Endoscopic vacuum therapy for intrathoracic anastomotic insufficiencies following oncological resections
Introduction
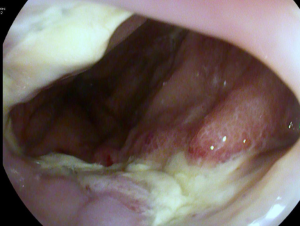
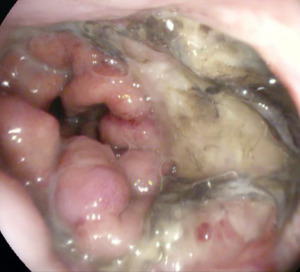
Anastomotic insufficiencies are severe events for patients after esophageal or gastric resections. Mediastinitis, pneumonia, esophageal-pulmonary fistulae, sepsis and death could result from intrathoracic anastomotic leaks (IAL). To diagnose IAL endoscopy is recommended. There is a wide spectrum of primary endoscopic findings in patients with IAL. Suspected findings for an IAL in the endoscopic view are exposed staplers, smeary coats, deficient perfusion of the anastomotic region, and secretion of fluids or pus via the anastomosis. Sure signs for IAL are apparent leaks and an accessible mediastinal cave. In Figures 1 and 2 different findings of IAL are shown. The absence of endoscopic pathological findings does not exclude an IAL.
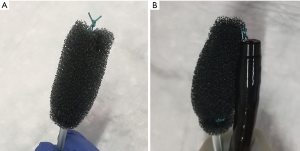
Historically EVT is a new evolving technique. In 2000 Weidenhagen and Gruetzner created a new endoscopic approach for treatment of rectal anastomotic insufficiencies (1). In brief, a perforated drainage is covered with open pore polyurethane sponge drainage (OPD). This open-pore drainage could be placed through the defect opening, so-called intracavitary, or directly within the lumen covering the defect zone completely, so-called intraluminary (2). The only commercially available OPD for esophageal EVT is the ESO-Sponge® (B. Braun, Melsungen AG, Melsungen, Germany). System and application modes are shown in Figures 3, 4 and 5. Outside the patient drain is connected to a vacuum system. For intrathoracic leaks the use of electric pumps is recommended because of automatically vacuum regulation.
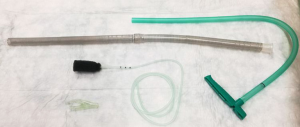
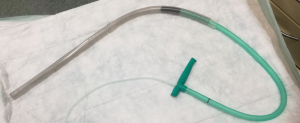
EVT offers a whole range of reported benefits for the endoscopic complication management after intrathoracic resections. We listed the advantages of EVT in Table 1.
Table 1
| Advantages of EVT in intrathoracic insufficiencies |
| Internal drainage of the abscesses without need of external drains |
| Treatment of insufficiencies not depended on localization |
| Downsizing of the wound cavity |
| Active wound cleansing |
| Stimulation of wound granulation |
| Removal of fluids (saliva, gastric and duodenal acids) from the anastomotic region |
| Compartmentalization of the wound cavity and prevention of further contamination |
| Drainage of the interstitial edema wound size reduction |
| Usable in cases of size incongruence of the anastomotic ends |
| Local sepsis control |
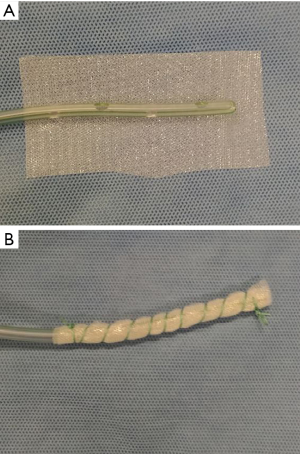
A new tool for endoscopic vacuum therapy (EVT) in IAL was introduced by Loske in 2017 (3). He wrapped the distal end of a gastric tube with a thin, double-layer drainage film (Suprasorb® CNP Drainage Film, Lohmann + Rauscher International GmbH and Co; Rengsdorf, Germany) named open-pore film-drainage (OFD). Resulted drains have got a very small diameter facilitating their introduction through the nares and placement into thin fistulas or in intraluminal position. If intestinal tubes are used for EVT with OFD and the gastric perforations are wrapped, patients could feed via the jejunal tube. Suction has to be established via the gastric tube. In Figure 6 preparation of an OFD on gastric tube is shown.
Methods
Literature database (Embase, PubMed, Medline) searched until July 2019 using the following thesaurus terms with no date restriction: IAL; insufficient esophageal anastomosis; endoscopic vacuum assisted closure (EVAC); endoscopic negative pressure wound therapy (ENPWT); endoscopic endoluminal vacuum therapy (EEVT); endoscopic negative pressure therapy (ENPT) and EVT. These search strategies were repeated using free text. References of all articles were searched to identify further relevant publications. All papers looking at the use of topical negative pressure in the management of esophageal leaks and perforations were reviewed. Series of fewer than five patients and individual case studies were excluded, as were animal studies. Where multiple papers described overlapping datasets the most recently published study was included.
Articles about treatment of anastomotic insufficiencies following esophagectomy or gastrectomy with intrathoracic anastomoses were included for the review. Studies about EVT for insufficient stapler lines following sleeve gastrectomy and non-surgical perforations were excluded. Patients with insufficient Myotomy of Zenker’s diverticulum were excluded in our analysis. Thirteen studies met the inclusion criteria for this review (Table 1), describing altogether 220 IAL patients. None of the studies were randomized or controlled, three studies reported prospective data collection.
In Table 2 all included publications are sorted by date of publication with number of IAL patients compared with all documented patients and corresponding success rates.
Table 2
| Reference | Year | Study type | Patients IAL | Patients all | Success rate IAL (%) | Success rate all (%) |
|---|---|---|---|---|---|---|
| Weidenhagen |
2010 | Case series | 6 | 6 | 100 | 100 |
| Brangewitz |
2013 | Retrospective cohort study | 28 | 32 | ? | 84.37 |
| Schniewind |
2013 | Retrospective cohort study | 14 | 17 | ? | 88.23 |
| Schorsch |
2014 | Case series | 21 | 35 | 95.24 | 91.43 |
| Mennigen |
2015 | Retrospective cohort study | 19 | 22 | 86.36 | 86.36 |
| Kuehn |
2016 | Case series | 11 | 21 | 81.82 | 90.48 |
| Laukoetter |
2017 | Retrospective cohort study | 39 | 52 | 89.74 | 88.46 |
| Mencio |
2018 | Retrospective cohort study | 2 | 15 | 100 | 100 |
| Berlth |
2019 | Retrospective cohort study | 51 | 77 | 80.39 | 77.92 |
| Pournaras |
2018 | Prospective cohort study | 7 | 21 | 100 | 95.24 |
| Noh |
2018 | Retrospective cohort study | 11 | 12 | 91.67 | 91.67 |
| Ooi |
2018 | Case series | 3 | 10 | 66.67 | 60 |
| Min |
2019 | Retrospective cohort study | 13 | 20 | ? | 95.00 |
| Total | – | – | 225 | 340 | ~89.2 | ~88.4 |
?, no data; ~, around.
Following items are analysed: EVT for first, second or third line strategy, delay between diagnosis of IAL and start of EVT, duration of therapy, number of endoscopic or surgical interventions, documented therapy success, documented complications, use of finished products or of self-manufactured components, use of electric pumps, enteral feeding during EVT, location of OPD or OFD, and competitive endoscopic procedures like dilatation or necrosectomy. Furthermore we review about therapy-related complications and if available follow-up data.
Results
Thirteen studies met the inclusion criteria for this review (Table 1), describing altogether 213 IAL patients. None of the studies were randomized or controlled. Three studies reported prospective data collections (10,13,15).
Working groups of Weidenhagen (4), Loske (17) and Brangewitz (5) published the first two case series of patients with postoperative intrathoracic leaks in 2010. Schorsch et al. (7) republished the early data of Loske within a larger patient cohort in 2014. Brangewitz et al. (5) reported a comparative study of therapy with EVT or stent placement in intrathoracic leaks. In this study success rate of EVT for IAL was not described. Schniewind et al. (6) performed a comparative, retrospective study on surgery, stent placement, EVT and conservative approach in IAL patients. We had to exclude three EVT patients of this study because of cervical anastomoses. Success rate for EVT treated patients was also not evident. In the retrospective cohort study of Laukoetter and colleagues (10) a precise analysis of EVT effectiveness for diverse etiologies of esophageal leaks was done. Berlth and Bludau et al. (12) published in 2018 a retrospective comparative analysis of EVT and SEMS, including 111 IAL patients over 10 years. Noh and colleagues (14) described in 2018 EVT treatment in 12 IAL patients. One case of perforation following to robotic esophageal diverticulectomy was excluded for this analysis. Ooi et al. (15) reported 2018 about their experiences with EVT in patients with esophageal leaks caused by different etiologies, 2 patients with IAL were included. In the recent publication of Min (16) 20 patients were treated with EVT for anastomotic insufficiencies. Because of cervical anastomoses 7 patients were excluded.
The time to primary diagnosis of IAL was documented in 8 studies (4,7-10,12,14,16) mean time was 10.19 days. Duration to the primary sponge placement following diagnosis was reported in 5 publications with a delay from 0 up to 39 days (4,10,12,14,16). Causes of insufficiencies (ischemia, primary material failure during the surgery) were not differentiated in the analyzed studies. In 5 reports EVT was (in part) the first therapeutic strategy (6-9,11). In these reports success rate of EVT for all patients was 92.1%. In case series with EVT as second or third line strategy (4,5,10,12-16) success rate was 87.55%. In publications with EVT as first line strategy (6-9,11), mortality rate was 7.27% in 8 patients (110 patients). In analysis with EVT for second or third line strategy (4,5,10,12-16), mortality rate increased up to 10.16% in 19 patients (187 patients).
In Table 3 details about EVT for first line strategy, duration of EVT, mortality rate, complications, and entired period of hospital stay are listed.
Table 3
| Reference | EVT first line | Complications (all patients, n) | Mortality rate (all patients) (%) | Duration of EVT (all patients) | Hospital stay (all patients) |
|---|---|---|---|---|---|
| Weidenhagen |
No | 0 | 16.67 | 20 days | 69.5 days |
| Brangewitz |
No | 3 | 15.62 | 23 days | 48.5 days |
| Schniewind |
Yes | ? | 11.76 | ? | 57 days |
| Schorsch |
Yes | 2 | 5.17 | 15.9 days | ? |
| Mennigen |
Yes | ? | 13.64 | 26.5 days | 58 days |
| Kuehn |
Yes | 2 | 4.76 | 12 days | ? |
| Laukoetter |
No | 2 | 9.61 | 20 days | 60 days |
| Mencio |
Yes | 0 | 0 | 27.5 days | ? |
| Berlth |
No | ? | 11.69 | 13.35 days | ? |
| Pournaras |
No | 2 | 14.29 | ? | 35 days |
| Noh |
No | 2 | 8.33 | 25 days | ? |
| Ooi |
No | 1 | 30 | 22 days | 62 days |
| Min |
No | ? | 5 | 14.5 days | 49 days |
| Total | 5/13 | ~16 | 11.27 | 19.97 days | 54.87 days |
?, no data; ~, around.
In 3 articles further therapeutic strategies were solely endoscopic approaches (4,5,8). Additionally surgical treatment or interventional treatment was documented in 2 case series (13,15).
In almost all reports sponge system was prepared by the endoscopists themselves. Use of electric vacuum pump is recommended in all publications. Documented vacuum preferences ranged from 70 (6) to 175 (11) mmHg, most frequent 125 mmHg (5,7-10,12-16).
Intracavitary or intraluminal position of the sponge was mentioned in 5 of the analysed studies (5,7,9,14,16). To place the sponge into the para-anastomotic cavity, a further dilatation of insufficiency was described in 3 reports (6,7,15). Complications related to dilatation were not documented.
Most studies consistently reported a change of sponges every 2–4 days. Noh et al. (14) reported some cases with very long changing intervals of up to 13 days. Comparison of the number of sponge changes related to first or second line EVT shows no differences (5.98 vs. 5.87 changes).
Enteral feeding during the EVT was specified in 4 studies with preferred enteral feeding tubes additionally to the EVT (4,10,12,13). In 1 study parenteral feeding was described for the whole time of EVT (15).
Mean period of EVT in IAL patients was 18.93 (12–27.5) days. Treatment success was variously defined as endoscopic or radiological closure of perforation with no evidence of ongoing sepsis.
It was not possible to extract the mortality rate of IAL patients in publications with mixed geneses of esophageal perforations and leaks. Scoring systems (CCI) to predict the risk of mortality was used in one publication (16). Clavien-Dindo-Classification (CDC) of surgical complications was used in 1 case series (9). In 9 publications adverse events were addressed (5,7,9-15). Most of these events were dislocations of the sponges, mucosal damages and bleedings. Duration of hospital stay of patients with IAL was named out in 9 publications (4-6,8,10,12,13,15,16) with a range of mean duration from 39 to 69 days.
Only in 3 studies (7,10,14) standardized follow-up examinations were reported, median follow-up was 17 months. In 2019 working group of Muenster reported a systematically follow-up of 25 EVT-patients in median 19.5 months after EVT in the upper GIT compared with 50 patients without EVT (18) in patients with non-complicated resections with anastomoses in the upper GIT. Dhayat et al. could show that most terms of a long-term QoL are equal in postsurgical patients treated with EVT and postsurgical patients without need of EVT.
Discussion
The major challenge for this review was to extract the number of EVT in IAL patients. In 7 publications EVT in postsurgical patients were examined (4-6,8,12,14,16), in 6 publications genesis of the leaks were mixed with iatrogenic or traumatic leaks (7,9-11,13,15).
Difficulties comparing intrathoracic EVT publications are: the high degrees of individuality in preparation of the sponge and drain, of sponge placement, the lack of information about further therapeutic strategies and pump systems with different settings. We could not found differences in therapeutic success or adverse events related to the adjusted vacuum.
The mean time to primary diagnosis of IAL was 10.19 (range, 8–17) days in searched articles. IAL patients are a cohort of seriously ill patients. They got IAL-related morbidities like pulmonary, kidney and liver failures. Early detection of IAL and goal-directed therapy could reduce these morbidities. In abnormalities of the post-surgical course following oncological resections with intrathoracic anastomoses early endoscopy to control the anastomosis should be performed.
We have to differentiate for EVT as first line strategy for IAL patients with fresh insufficiencies and for secondary or third line strategies in patients in severe situations with multiple organ failure. It was shown that EVT for first line strategy reduce mortality rate.
Definition of therapeutic success was not given in 8 of 13 studies. So some compromises had to be accepted in this review. Depending on the study type, it was not possible to determine the success rate for IAL patients in all publications. Study designs were not clarified in some releases. Furthermore, reporting of complications was inconstant and there were varying definitions of therapeutic success. For some of our attentions, like strategy of enteral feeding or placement of the sponge system in IAL patients, there was no information.
Taking into account all published series with experience of >5 patients, we calculated an overall success rate of EVT for anastomotic leakage after esophagectomy/gastrectomy of nearly 90% in more than 210 patients.
Some technical aspects are rarely mentioned like dilatation of IAL to pass with the endoscope into the mediastinal cave in 3 reports. Small opening could exam with a transnasal gastroscope, if a large wound cavity or abscess is diagnosed, a dilatation and intracavitary OPD placement is possible.
The most frequent described adverse events are sponge dislocation, minor bleeding after sponge exchange due to ingrowth of granulation tissue into the sponge, and anastomotic strictures. Most relevant complications depended on EVT are major bleeding events. Laukoetter et al. (10) reported 2017 in a prospective cohort of 52 patients with mixed causes of esophageal leaks about 2 fatal major bleeding events. Authors recommend EVT for esophageal perforations to be performed combined with a CT scan of the thorax done directly before or after every first endoscopic placement of the sponge to exclude close proximity of the sponge to cardiovascular structures with subsequent risk of erosion bleeding. Fatal bleedings occurred only in patients with intracavitary OPD placement.
Enteral feeding is recommended for all post-surgical patients (19). Especially in patients after intrathoracic resections enteral feeding is important because of low pre-therapeutic nutritional status of these patients. Hébuterne et al. reported in 2014 a prevalence of malnutrition in patients with esophagus and/or stomach cancer up to 60.2% (20). To ensure enteral feeding use of OFD on intestinal tubes or placement of PEG are possible.
To examine EVT in IAL patients in multicenter studies it would be desirable to standardize particular therapeutic parts like enteral feeding, level of applied vacuum, period between endoscopic procedures and criteria of healing success.
To sum up, EVT seems to achieve high success rates especially in patients with IAL. It is important to keep a couple of aspects in mind: (I) perform an early endoscopy to evaluate intrathoracic anastomosis; (II) use EVT as first line strategy in IAL; and (III) ensure enteral feeding in IAL patients. Because of by short intervals of changing sponge system evaluation of the healing process is ensured.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoe.2019.08.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Weidenhagen R, Gruetzner KU, Wiecken T, et al. Endoscopic vacuum-assisted closure of anastomotic leakage following anterior resection of the rectum: a new method. Surg Endosc 2008;22:1818-25. [Crossref] [PubMed]
- Loske G, Muller C. Endoscopic vacuum-assisted closure of upper intestinal anastomotic leaks. Gastrointest Endosc 2009;69:601-2; author reply 602. [Crossref] [PubMed]
- Loske G, Liedke M, Schloricke E, et al. Endoscopic negative-pressure therapy for duodenal leakage using new open-pore film and polyurethane foam drains with the pull-through technique. Endoscopy 2017;49:E300-E302. [Crossref] [PubMed]
- Weidenhagen R, Hartl WH, Gruetzner KU, et al. Anastomotic leakage after esophageal resection: new treatment options by endoluminal vacuum therapy. Ann Thorac Surg 2010;90:1674-81. [Crossref] [PubMed]
- Brangewitz M, Voigtlander T, Helfritz FA, et al. Endoscopic closure of esophageal intrathoracic leaks: stent versus endoscopic vacuum-assisted closure, a retrospective analysis. Endoscopy 2013;45:433-8. [Crossref] [PubMed]
- Schniewind B, Schafmayer C, Voehrs G, et al. Endoscopic endoluminal vacuum therapy is superior to other regimens in managing anastomotic leakage after esophagectomy: a comparative retrospective study. Surg Endosc 2013;27:3883-90. [Crossref] [PubMed]
- Schorsch T, Muller C, Loske G. Endoscopic vacuum therapy of perforations and anastomotic insufficiency of the esophagus. Chirurg 2014;85:1081-93. [Crossref] [PubMed]
- Mennigen R, Harting C, Lindner K, et al. Comparison of Endoscopic Vacuum Therapy Versus Stent for Anastomotic Leak After Esophagectomy. J Gastrointest Surg 2015;19:1229-35. [Crossref] [PubMed]
- Kuehn F, Schiffmann L, Janisch F, et al. Surgical Endoscopic Vacuum Therapy for Defects of the Upper Gastrointestinal Tract. J Gastrointest Surg 2016;20:237-43. [Crossref] [PubMed]
- Laukoetter MG, Mennigen R, Neumann PA, et al. Successful closure of defects in the upper gastrointestinal tract by endoscopic vacuum therapy (EVT): a prospective cohort study. Surg Endosc 2017;31:2687-96. [Crossref] [PubMed]
- Mencio MA, Ontiveros E, Burdick JS, et al. Use of a novel technique to manage gastrointestinal leaks with endoluminal negative pressure: a single institution experience. Surg Endosc 2018;32:3349-56. [Crossref] [PubMed]
- Berlth F, Bludau M, Plum PS, et al. Self-Expanding Metal Stents Versus Endoscopic Vacuum Therapy in Anastomotic Leak Treatment After Oncologic Gastroesophageal Surgery. J Gastrointest Surg 2019;23:67-75. [Crossref] [PubMed]
- Pournaras DJ, Hardwick RH, Safranek PM, et al. Endoluminal Vacuum Therapy (E-Vac): A Treatment Option in Oesophagogastric Surgery. World J Surg 2018;42:2507-11. [Crossref] [PubMed]
- Noh SM, Ahn JY, Lee JH, et al. Endoscopic Vacuum-Assisted Closure Therapy in Patients with Anastomotic Leakage after Esophagectomy: A Single-Center Experience. Gastroenterol Res Pract 2018;2018:1697968 [Crossref] [PubMed]
- Ooi G, Burton P, Packiyanathan A, et al. Indications and efficacy of endoscopic vacuum-assisted closure therapy for upper gastrointestinal perforations. ANZ J Surg 2018;88:E257-E263. [Crossref] [PubMed]
- Min YW, Kim T, Lee H, et al. Endoscopic vacuum therapy for postoperative esophageal leak. BMC Surg 2019;19:37. [Crossref] [PubMed]
- Loske G, Schorsch T, Muller C. Endoscopic vacuum sponge therapy for esophageal defects. Surg Endosc 2010;24:2531-5. [Crossref] [PubMed]
- Dhayat SA, Schacht R, Mennigen R, et al. Long-Term Quality of Life Assessment After Successful Endoscopic Vacuum Therapy of Defects in the Upper Gastrointestinal Tract Quality of Life After EVT. J Gastrointest Surg 2019;23:280-7. [Crossref] [PubMed]
- Seres DS, Valcarcel M, Guillaume A. Advantages of enteral nutrition over parenteral nutrition. Therap Adv Gastroenterol 2013;6:157-67. [Crossref] [PubMed]
- Hébuterne X, Lemarie E, Michallet M, et al. Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN J Parenter Enteral Nutr 2014;38:196-204. [Crossref] [PubMed]
Cite this article as: Wichmann D, Schempf U, Mothes B, Stüker D, Königsrainer A, Schweizer U, Werner CR. Endoscopic vacuum therapy for intrathoracic anastomotic insufficiencies following oncological resections. Ann Esophagus 2019;2:16.