
The effect of oral sucralfate on postprandial proximal gastric acid pocket
Introduction
The increased number of acidic gastroesophageal reflux episodes in the postprandial period is at odds with the intuitive idea of meals buffering gastric acid. The concept of a postprandial proximal gastric acid pocket (PPGAP) solved this apparent paradox. In 2001, Fletcher et al. (1) showed the presence of acid at the gastroesophageal junction that is not neutralized by food. In the following years, other groups also clearly demonstrated that gastric acid is not uniformly spread in the stomach in the postprandial period (2-6).
The PPGAP is thought to be a reservoir of non-neutralized acid surrounding the gastroesophageal junction that enables the occurrence of postprandial acidic reflux events when the distal stomach is alkaline due to meal’s buffering effect (5). Interestingly, some researchers demonstrated that the refluxate may be more acidic than distal gastric content, thus corroborating the PPGAP as the source of these acidic postprandial reflux events (1-5).
It has been hypothesized that the PPGAP is the result of secreted acid mixing inadequately with gastric contents after meals and remaining separate from the non-acidic chyme. This phenomenon might be due to decreased proximal gastric motor activity in the postprandial period and food components partitioning in the stomach (1).
The existence of acid at the gastroesophageal junction after meals modified our current understanding about gastroesophageal junction diseases. For instance, PPGAP may represent an alternative explanation for epithelial damage at the gastroesophageal junction in individuals that gastroesophageal reflux disease (GERD) could not be diagnosed by routine work-up (7). Some authors also believe that PPGAP may have an intrasphincteric and even intraesophageal component, behaving like a film, not a pocket (5). The pocket theory implies that, after meals, a volume of unbuffered acid floats on top of the non-acid chyme. The film theory suggests that unbuffered acid remains as an acid layer attached to the acid-secreting mucosa whereas meals are localized in the central area of gastric lumen.
This study attempts to expand current knowledge about PPGAP, especially on the morphological aspect (film vs. pocket). The rational basis for using sucralfate to determine PPGAP tridimensional structure was the hypothesis that mucosal coating with sucralfate would probably not change acid layer configuration in case of a pocket whereas an acid film, devoid of significant volume, would more likely be modified by this drug. In addition, this study aims to assess whether sucralfate would be a suitable potential drug targeting specifically the PPGAP.
Methods
Population
Twenty-six patients investigated for GERD were prospectively studied.
All patients had GERD symptoms and underwent an upper digestive endoscopy.
Exclusion criteria were previous foregut operation and denial to participate in the study.
Demographic and endoscopic data are shown in Table 1.
Table 1
| Variables | Data |
|---|---|
| Age (years) | 51 [45–60] |
| Gender | 19 female/7 male |
| Esophageal symptoms (heartburn/regurgitation) | 24 (92.3%) |
| Endoscopic data | |
| Hiatal hernia | 15 (57.7%) |
| Esophagitis | 14 (53.8%) |
| Barrett’s esophagus | 1 (3.8%) |
Data are expressed as median and interquartile ranges or n (%).
High-resolution manometry (HRM)
All patients fasted for 8 hours before the tests. All participants underwent HRM (Medtronic, Los Angeles, CA, USA) to determine lower esophageal sphincter (LES) borders.
pH monitoring
After HRM, all individuals underwent gastroesophageal pH studies (Alacer Biomedica, São Paulo, SP, Brazil).
Antacid medications were discontinued opportunely.
The pH catheter was initially placed in the proximal stomach 5 cm below LES lower border (LESLB). A station pull-through was performed from 5 cm below LESLB in increments of 1cm signaled by pushing the event bottom until detection of gastric-to-esophageal pH transition point or until LESUB was reached, according to classical methodology for PPGAP detection (1).
After this first pull-through in the fasting state, the sensor was replaced at the initial position (5 cm below LESLB) and the patients received a standardized fatty meal (hamburger, 11% fat and chocolate milk, 3% fat). The pull-through was identically repeated 10 minutes after food intake was finished.
Patients with a detected PPGAP received 2 g/10 mL of oral sucralfate suspension (Sucrafilm®, EMS, Brazil) and underwent a third pull-through for PPGAP detection 10 minutes after administration of the mucosal coating agent.
After the protocol for PPGAP evaluation, all individuals completed conventional ambulatory 24-hour pHmonitoring.
PPGAP assessment
PPGAP was defined by the presence of an acid reading (pH <4) in a segment of the proximal stomach between nonacid segments distally (food) and proximally (gastric-to-esophageal pH transition point) (8) (Figure 1). PPGAP length and position relative to LESLB were recorded postprandially and after sucralfate.
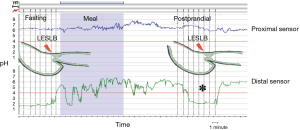
Ethics
The protocol was approved by the Institutional Review Board of Federal University of Sao Paulo (#13473013.6.0000.5505) and was conducted in accordance with the Helsinki Declaration of 1964 (revised 2008). Informed consent was obtained from all individuals.
The authors are responsible for the manuscript and no professional or ghost writers were hired.
Results
Esophageal manometry and pHmonitoring
Esophageal manometry parameters and prolonged ambulatory pHmonitoring results are expressed in Table 2.
Table 2
| Variables | Data |
|---|---|
| High-resolution manometry data | |
| % hypotonic LES | 17/26 (65.4%) |
| LES basal pressure (mmHg) | 7.1 (2.8–20.3) |
| % short LES | 21/26 (80.8%) |
| LES length (mm) | 20 [18–26] |
| Dissociation between HPZ intrinsic and extrinsic components | 14/26 (53.8%) |
| Distance between LES and crural diaphragm (mm) | 16.5 (9.2–29) |
| Distal esophageal wave amplitude (mmHg) | 60.8 (49.4–92.9) |
| pH monitoring data | |
| Abnormal esophageal acid exposure (at 5 cm above LES) | 21/26 (80.8%) |
| DeMeester score | 37 (12.6–48.1) |
Data are expressed as median and interquartile ranges. LES, lower esophageal sphincter; HPZ, high pressure zone.
Gastric pH
Four patterns previously described of gastric acidity (9) were identified: permanent alkaline stomach in 4 out of 26 patients (15%); no PPGAP detected in 5 out of 26 (19%); permanent acid stomach (no buffering effect of food) in 6 out 26 (23%) and PPGAP present in 11 out of 26 (43%) (Figure 2).
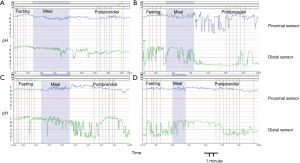
PPGAP
PPGAP was detected in 11 out of 26 (43%) patients. Intrasphincteric extension of the PPGAP was noticed in 3 out of 11 (27%) cases.
After sucralfate, PPGAP disappeared (or it was displaced distally beyond detection) in 3 out of 11 patients (27%) and was unaltered in 1 of the 11 (9%). PPGAP length increased in 5 out of 11 (45%) of the individuals and a decrease in 2 out of 11 (18%). After sucralfate, intrasphincteric extension of the PPGAP disappeared in patients with this finding previously but it was present de novo in 1 out of 11 (9%).
Figure 3 shows pHmonitoring traces of different sucralfate effects on PPGAP, summarized in Figure 4.
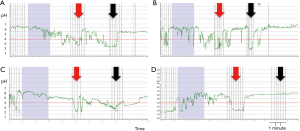

Discussion
Our results show that sucralfate affected PPGAP in most cases (91%) with an increase in length in almost half of the patients and a decrease/suppression in the other half.
PPGAP morphology
It is still controversial whether acidity at the gastroesophageal junction would be better described as an acid pocket or an acid film (6). In favor of the film concept is the presence of an intrasphincteric extension of the PPGAP measured by pH monitoring, even with an intact LES (6,10,11) or increased LES basal pressure with the use of baclofen (12). In favor of the pocket concept is the presence of a volume of acid not limited to the gastric wall as detected by magnetic resonance or scintigraphy (13,14).
Sucralfate is a sulfated aluminum salt of sucrose. This non-systemic agent does not have any acid-neutralizing effect and does not influence gastric acid secretion as well (15,16). It is a mucosal-coating agent that attaches both to injured and normal gastric mucosa (15). We theorized that mucosal coating with a non-acid substance would alter normal PPAGP morphology in case of a film. On the other hand, a tridimensional pocket would be undisturbed by mucosal coating. In our study, sucralfate altered the acid pocket in 91% of the patients favoring the presence of an acid film as an important component of the PPGAP. Furthermore, the presence of an intrasphincteric PPGAP was suppressed by sucralfate. However, the finding of an increase in length in half of the patients may suggest a dual behavior of the PPGAP as a film and a pocket.
If our individual results are carefully analyzed, an erratic response to sucralfate administration was found. Although PPGAP extinction was found only in patients GERD—and in the absence of a hiatal hernia, the presence of GERD and hiatal hernia was unpredictable in the other patterns. Interesting only is that in PPAGP increase group, 4 out of 5 were GERD patients. In this group the acid pocket continued to form after ten minutes, and sucralfate was far from able to suppress it. Intrasphincteric extension of PPGAP also does not predict sucralfate effect.
PPGAP treatment
A therapy directed towards PPGAP may be useful in patients with postprandial symptoms and a putative prevention of carditis and Barrett’s esophagus as a consequence of a permanently acidic environment adjacent to the esophagogastric junction (16).
Gastric acid output blocking by proton pump inhibitors has shown to decrease PPGAP volume, acidity and symptoms but did not suppressed PPGAG (4,17-19). This may be caused by accumulation of exogenous acid from the food or small amounts of acid still secreted despite pharmacological blockage and concentrated in the PPGAP. Acceleration of gastric emptying with prokinetics seems to displace the PPGAP distally avoiding reflux of the acid within the PPGAP (4). The same effect was observed with an alginate-antacid formulation by forming a gel raft on top of the acidic layer (11). A fundoplication (9) decreases the incidence of the PPGAP by changes in gastric anatomy.
Sucralfate promoted suppression of the PPGAP in a quarter of the patients and a decrease in length in other 20%. This result is suboptimal and not different from previous described therapies. Curiously, all patients in which sucralfate extinguished PPGAP were non-refluxers without a hiatal hernia.
Study limitations
This study comprises a small number of patients, since PPGAP was not found to be as ubiquitous as previously described (1,2,4,6,14). A control group without sucralfate administration was not studied. This group may be argued valuable to differentiate the effect of the drug from washout of the acid. Previous studies; however, showed that PPGAP may be present even 120 minutes after a meal (12,20). Furthermore, the results of previous studies, including from our group, fill the need for a control group. The timepoint to assess whether the acid pocket was present or not was 10 minutes based on the methodology of previous studies (8). It has been shown that it may take longer to fully form; therefore our protocol may have excluded a subset of patients. The main aim of the study; however, was to investigate patients with proved PPGAP, not access the incidence of the condition.
Future studies in combination with magnetic resonance or scintigraphy may shed more light in the pathophysiology of the PPGAP as the findings of the current study are not definite proof of PPGAP spatial configuration but a modest contribution.
Conclusions
We conclude that the fact that sucralfate altered the PPGAP configuration in up to 91% of the patients, suggests that acidity at the gastroesophageal junction may have a film component, since a mucosal coating drug would probably not disturb an acid pocket. Also, sucralfate is not an adequate target therapy for the PPGAP.
Acknowledgments
We are indebted to Ms. Vanessa Horich Tuxen for her invaluable assistance with the esophageal tests.
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoe.2018.11.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The protocol was approved by the Institutional Review Board of Federal University of Sao Paulo (#13473013.6.0000.5505) and was conducted in accordance with the Helsinki Declaration of 1964 (revised 2013). Informed consent was obtained from all individuals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fletcher J, Wirz A, Young J, et al. Unbuffered highly acidic gastric juice exists at the gastroesophageal junction after meal. Gastroenterology 2001;121:775-83. [Crossref] [PubMed]
- Hila A, Bouali H, Xue S, et al. Postprandial stomach contents have multiple acid layers. J Clin Gastroenterol 2006;40:612-7. [Crossref] [PubMed]
- Simonian HP, Vo L, Doma S, et al. Regional postprandial differences in pH within the stomach and gastroesophageal junction. Dig Dis Sci 2005;50:2276-85. [Crossref] [PubMed]
- Vo L, Simonian HP, Doma S, et al. The effect of rabeprazole on regional gastric acidity and the postprandial cardia/gastro-oesophageal junction acid layer in normal subjects: a randomized, double-blind, placebo-controlled study. Aliment Pharmacol Ther 2005;21:1321-30. [Crossref] [PubMed]
- Clarke AT, Wirz AA, Seenan JP, et al. Paradox of gastric cardia: it becomes more acidic following meals while the rest of stomach becomes less acidic. Gut 2009;58:904-9. [Crossref] [PubMed]
- Pandolfino JE, Zhang Q, Ghosh SK, et al. Acidity surrounding the squamocolumnar junction in GERD patients: "acid pocket" versus "acid film". Am J Gastroenterol 2007;102:2633-41. [Crossref] [PubMed]
- Kahrilas PJ, McColl K, Fox M, et al. The acid pocket: a target for treatment in reflux disease? Am J Gastroenterol 2013;108:1058-64. [Crossref] [PubMed]
- Herbella FA, Vicentine FP, Silva LC, et al. Postprandial proximal gastric acid pocket and gastroesophageal reflux disease. Dis Esophagus 2012;25:652-5. [Crossref] [PubMed]
- Herbella FA, Vicentine FP, Del Grande JC, et al. Postprandial proximal gastric acid pocket in patients after laparoscopic Nissen fundoplication. Surg Endosc 2011;25:3198-201. [Crossref] [PubMed]
- Fletcher J, Wirz A, Henry E, et al. Studies of acid exposure immediately above the gastro-oesophageal squamocolumnar junction: evidence of short segment reflux. Gut 2004;53:168-73. [Crossref] [PubMed]
- Kwiatek MA, Roman S, Fareeduddin A, et al. An alginate-antacid formulation (Gaviscon Double Action Liquid) can eliminate or displace the postprandial 'acid pocket' in symptomatic GERD patients. Aliment Pharmacol Ther 2011;34:59-66. [Crossref] [PubMed]
- Scarpellini E, Boecxstaens V, Farré R, et al. Effect of baclofen on the acid pocket at the gastroesophageal junction. Dis Esophagus 2015;28:488-95. [Crossref] [PubMed]
- Goetze O, Treier R, Fox M, et al. The effect of gastric secretion on gastric physiology and emptying in the fasted and fed state assessed by magnetic resonance imaging. Neurogastroenterol Motil 2009;21:725-e42. [Crossref] [PubMed]
- Beaumont H, Bennink RJ, de Jong J, et al. The position of the acid pocket as a major risk factor for acidic reflux in healthy subjects and patients with GORD. Gut 2010;59:441-51. [Crossref] [PubMed]
- Vaira D, Corbelli C, Brunetti G, et al. Gastric retention of sucralfate gel and suspension in upper gastrointestinal diseases. Aliment Pharmacol Ther 1993;7:531-5. [Crossref] [PubMed]
- Guth PH. Mucosal coating agents and other nonantisecretory agents. Are they cytoprotective? Dig Dis Sci 1987;32:647-54. [Crossref] [PubMed]
- Steingoetter A, Sauter M, Curcic J, et al. Volume, distribution and acidity of gastric secretion on and off proton pump inhibitor treatment: a randomized double-blind controlled study in patients with gastro-esophageal reflux disease (GERD) and healthy subjects. BMC Gastroenterol 2015;15:111. [Crossref] [PubMed]
- Rohof WO, Bennink RJ, de Ruigh AA, et al. Effect of azithromycin on acid reflux, hiatus hernia and proximal acid pocket in the postprandial period. Gut 2012;61:1670-7. [Crossref] [PubMed]
- Morgan D, Pandolfino J, Katz PO, et al. Clinical trial: gastric acid suppression in Hispanic adults with symptomatic gastro-oesophageal reflux disease - comparator study of esomeprazole, lansoprazole and pantoprazole. Aliment Pharmacol Ther 2010;32:200-8. [Crossref] [PubMed]
- Scarpellini E, Boecxstaens V, Broers C, et al. Effect of baclofen on gastric acid pocket in subjects with gastroesophageal reflux disease symptoms. Dis Esophagus 2016;29:1054-63. [Crossref] [PubMed]
Cite this article as: Silva LC, Herbella FAM. The effect of oral sucralfate on postprandial proximal gastric acid pocket. Ann Esophagus 2018;1:20.


