
Collis gastroplasty: why, when and how?
Introduction
John Leigh Collis (1911–2003) was a British cardiothoracic surgeon whose name today is primarily associated with the so-called “Collis gastroplasty”, which he first described in 1957 after having performed the procedure on 9 patients. This gastroplasty was designed to alleviate the increasing incidence of the condition of short esophagus (SE) as associated with gastroesophageal reflux disease (GERD) and hiatal hernias. Collis’ original description was of a technique which caused a functional “lengthening of the esophagus” by performing a vertical gastroplasty in order to create a tubular length of neo-esophagus created from the gastric fundus. The original operation did not include a fundoplication (1). Later, a transthoracic fundoplication was added to the Collis gastroplasty in order to correct the underlying disease, and probable cause of the shortening itself.
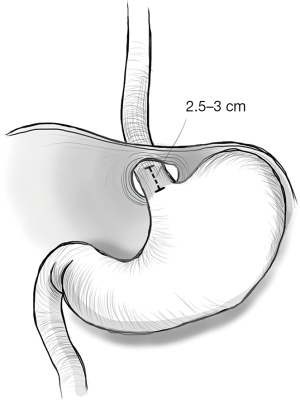
SE is defined as a gastroesophageal junction (GEJ) that, even after extensive surgical mobilization, does not lie more than 2.5 cm below the diaphragmatic hiatus without undue tension (2). The pathophysiology underlying this finding is most probably multifactorial. In the case of GERD, the chronic inflammation caused by acid or non-acid reflux on the muscular wall of the esophagus is considered the main cause for shrinking and shortening of the esophagus. In fact, depending on the site exposed to the chronic regurgitation, the related formation of fibrosis can lead to circumferential strictures (if the circular muscular layer is most damaged) or to a shortening of the esophagus, if the longitudinal layers are mainly involved. Other inflammatory conditions, such as caustic ingestion, sclerodermia or inflammatory bowel disease (IBD) (Crohn’s disease in particular) or paraesophageal hernias are more frequently related to SE. The exact incidence of a ‘true SE’ in the literature is very heterogeneous, ranging from 60% (3), especially in open series, to 0% in some series (4-6). Studies coming from high volume minimally invasive surgery (MIS) centers documents that between 3% to 20% of patients will need a procedure to lengthen the esophagus following an extensive mediastinal dissection (2,7-10).
Awais and colleagues (11) showed that, in 275 patients scheduled for redo antireflux surgery, 64% of the patients had failure of the first operation because of transmediastinal migration of the fundoplication or recurrent hiatal hernia possibly related to tension from a SE. During the reintervention, even after a complete mediastinal mobilization, 43% of the patients in their series needed a Collis gastroplasty.
When do we treat
Prompt recognition of the SE is a key to a successful antireflux surgery. In fact, failure to identify this pathologic finding favors the subsequent displacement of a fundoplication. Such a herniation of the wrap into the intrathoracic space is thought to be responsible for surgical failure of reflux treatment in one fifth to one third of cases, whether it was an open or laparoscopic approach.
Although preoperative tests [barium X-ray (RX), esophagogastricduodenoscopy (EGDS), manometry, among others] can provide some preoperative indication of the SE, it is only at the time of operation that a definitive diagnosis of SE can be made.
A review from Swanstrom et al. studied the preoperative indicators that are predictive of the presence of a SE at the time of surgery (12,13). Some are linked to the history of the patient, such as a long history of GERD and of previous failed antireflux procedures. Other criteria are related to the results of preoperative exams, such as alteration at the lower esophageal sphincter (LES) (e.g., no high-pressure zone, distal hypo- or aperistalsis) visible with high resolution manometry (HRM); the presence of the GEJ > than 5 cm above the hiatus, signs of severe esophagitis (grades III–IV), Barrett’s dysplasia, presence of peptic ulcers at the EGDS and finally the presence at the barium swallow of 5 cm or more, non-reducible type I hiatal hernia, a giant type III hiatal hernia or strictures. The presence of these criteria should raise the high suspicion on the presence of a SE (12,13). Unfortunately, none of these criteria are currently highly sensitive nor specific and no predictive score of primary surgery failure currently exists.
Yano and colleagues have proposed a new index: the esophageal length index (ELI) as a predictive scoring system (14). The ELI is derived from the ratio between the esophageal length, measured from the incisors to the GEJ (in centimeters), and the height (in meters) of the patient. The index was calculated by performing a retrospectively analysis of more than 280 patients operated for antireflux surgery, with or without a lengthening procedure. They found that patients had an 83% negative predictive value to have a SE if their ELI was higher than 19.5. The specificity was 95%. The primary drawback of this as well as other predictive scales is that they are never 100% specific and therefore the surgeon must always be prepared to deal with a shortened esophagus if performing a type II surgical mobilization of the esophagus up until the aortic arch in the mediastinum fails to achieve adequate intra-abdominal length. Therefore, it is worth having knowledge of lengthening techniques or seeking an expert evaluation when high suspicion of SE is present.
How do we treat?—operative techniques
Since 1957 many different techniques have been described to lengthen the esophagus. During the open era most of them required a thoracotomy. This was because of the difficulties in mobilizing the esophagus up in the mediastinum working from the abdomen. On the other hand, laparoscopic techniques have mostly eliminated the need for thoracotomy.
As MIS is nowadays the standard of care even in redo surgery, with similar if not better results than open techniques, we will focus on minimally invasive approaches to the SE.
There are five MIS operations that have been described to lengthen the esophagus. For the most part, the operative set up does not change if a SE is suspected or not.
For laparoscopic antireflux surgery, it is almost always necessary to mobilize the distal part of the esophagus above the hiatus for at least 3–4 cm in order to perform the fundoplication. If this mobilization is not enough to have a tensionless 2.5 to 3 cm esophageal tract between the GEJ and the hiatus, a more extensive transhiatal dissection, type 2 mediastinal dissection, even up to the aortic arch, is performed. This procedure will result in an adequate intraabdominal esophageal length in most patients.
A Collis gastroplasty is indicated if neither a standard or extended type 2 dissection results in sufficient esophageal tissue to perform fundoplication without residual axial tension on the gastroesophageal tube (Figure 1).
The main procedures are the following:
- Thoraco-laparoscopic: (I) 1996 Swanstrom right transthoracic stapler approach (2); (II) 2000 Awad left thoracoscopic transthoracic stapler approach (15).
- Total laparoscopic: (I) 1998 Johnson total laparoscopic circular stapler technique (16); (II) 2004 Wedge fundectomy (WF), Terry et al. (7).
The first procedure, described by Swanstrom et al. in 1996 (2), consists of a combined laparoscopic—thoracoscopic approach. The first part of the operation, which included the complete mobilization of the mediastinal esophagus, is performed using a standard laparoscopic approach for fundoplication. In cases where a Collis gastroplasty is needed, the right thoracic field is prepared. During this step of the operation, a second laparoscope is inserted through a trocar placed at the level of the anterior axillary line at the IV intercostal space. The right lung is collapsed by use of positive pressure CO2 insufflation of the right chest to 10 mm/Hg. Using the 0° laparoscope, the path towards the esophageal hiatus is identified under direct visualization and a small incision is made at the level of the right parietal pleura from the abdominal side. The laparoscope is then removed and a straight 45- or 60-mm endoscopic stapler with a blue cartridge is carefully advanced while keeping the trocar in the same direction. A 45 French esophageal dilator is placed and the previously mobilized greater curve of the stomach is rotated anteriorly and worked into the open jaws of the stapler (the stapler being angulated parallel to the esophagus and lesser curvature of the left side of the esophagus. One charge is sufficient to produce a 3–6 cm esophageal lengthening at the level of the His angle.
The second technique, described in 2000 by Awad et al. (15), is conceptually very similar to the first one, except the approach is performed via the left chest and an articulating 12 mm stapler is used. In this case, the articulating stapler is directly pushed from the port to the mediastinum once thoracoscopy has determined there are no adhesions or obstructions between the port site and the posterior mediastinal sulcus. For the most part, a second laparoscope is not needed for guidance. After the diaphragm indentation is recognized from the abdomen, the tip of the stapler is slid over the diaphragm towards the hiatus. When it enters the abdominal cavity, the jaws are rotated towards the patient’s left side. The stapler is then positioned at the angle of His and a neo-esophagus is created using a 30-, 45- or 60-mm blue load stapler depending on the added length needed. A 45 Fr bougie is again used to size the neo-esophagus (Figure 2).
Two other techniques do not use thoracoscopy to lengthen the esophagus.
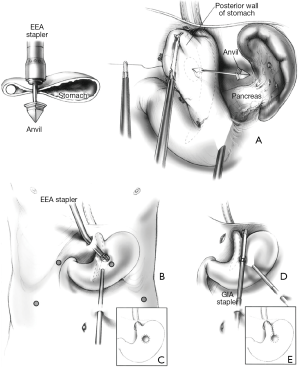
The oldest one, proposed by Johnson and his colleagues in 1998 (16), is the laparoscopic version of the circular stapler technique introduced by Steichen in open surgery in 1986. In order to perform the operation, an entry point on the gastric wall is marked, 3 cm inferior to the angle of His, for insertion of the anvil trocar of a 25 mm circular stapler. An upper abdominal port site is widened to allow the insertion of the circular stapler itself which is coupled to the anvil and fired adjacent to a 48 Fr bougie. Once this hole is made, a linear stapler is inserted through a lower abdominal port, opened and introduced through the hole and parallel to the main esophageal axis up to the angle of His and a vertical staple line is created (Figure 3). The complexity of this approach and its necessity of multiple staplers has made it of mostly historical interest only.
Another fully laparoscopic approach was proposed by Terry in 2004 (7). It involves the use of only articulating linear staplers. The port setting is the same used for a standard laparoscopic fundoplication.
After mobilization of the distal esophagus, if a gastroplasty is indicated, the upper gastric fundus is mobilized and a 45–48 Fr bougie is placed in the esophagus and stomach. The left upper most laparoscopic port is converted to a 12 mm port. A point 3 cm below the angle of His and next to the margin of the bougie is marked and an articulated stapler is introduced from the upper left port. The stapler is fired 1 to 3 times until the mark adjacent to the bougie is reached following a perpendicular pathway starting from the greater curvature. Once reaching the mark, another stapler is oriented upwards and parallel to the esophagus and bougie and a final staple line is created (Figure 4). The resulting “wedge” of gastric fundus is then removed.

Results
Failure rates for primary antireflux procedures range from 2–30% and approximately 3–6% of patients who undergo primary fundoplication will need complex redo operations to recover from failed primary intervention. It has been proposed that a significant percentage of these failures are due to axial tension on the repair from inappropriately addressed shortening of the esophagus (11). While “the SE” remains a somewhat controversial issue—with one extreme arguing that it is clinically irrelevant and the other extreme arguing that almost 50% of hiatal hernia patients should have a lengthening procedure—it is undeniable that chronic reflux disease can lead to foreshortening of the esophagus. Most high-volume reflux centers feel that while SE exists and is fairly common, it can usually be dealt with by extended mediastinal dissection mediastinal mobilization. Most high-volume centers would also agree that on occasion—perhaps in 2–5% of cases—mobilization is not enough and a lengthening procedure is needed. Fortunately, there are good minimally invasive options to achieve a tension free repair. Of the options we detail, the “WF” and the left thoracoscopic Collis (LTC) are currently the most commonly performed.
Both minimally invasive gastroplasty techniques have documented high technical success rates (87–91% for LTC and 90% for WF). With regard to reflux symptoms, symptomatic control is uniformly excellent (90–100%, 89–100%) and patients are overall pleased with results (7,9,10,18,19). Unfortunately, physiologic results are more complex as the Collis procedure is truly a compromise. The neo-esophagus segment is relatively motile, which can lead to dysphagia. Additionally, conduit size discrepancy can lead to issues (progressive dilation if too wide/dysphagia if too narrow) and the ectopic gastric mucosa can continue to secrete acid and lead to a localized esophagitis (20,21). This means that as many as 52% of post Collis/fundoplications may need to be on long-term acid suppression (21). This disappointing fact must be taken in the context of two things: clinically Collis patients are overall satisfied with their results and the alternatives for this non-physiologic solution are worse—including esophagectomy or total gastrectomy.
Conclusions
Collis gastroplasty is a long-standing surgical solution to the relatively rare finding of the axial shortened esophagus. In the MIS era, the SE remains a somewhat controversial subject, with some arguing it is more frequent than suspected and is responsible for high recurrence rates in specific patient groups. The vast majority of high-volume centers believe that the SE is a true entity, but fortunately in the proton pump inhibitor (PPI) era is relatively rare. None the less, when it is encountered during antireflux surgery, it must be effectively addressed or the repair will be subject to axial tension and high failure rates. Unfortunately, it is a rather non-physiologic solution and may commit patients to continued need for acid suppressive medication.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Luigi Bonavina) for the series “Gastroesophageal Reflux Disease” published in Annals of Esophagus. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoe.2018.09.01). The series “Gastroesophageal Reflux Disease” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- COLLIS JL. An operation for hiatus hernia with short oesophagus. Thorax 1957;12:181-8. [Crossref] [PubMed]
- Swanstrom LL, Marcus DR, Galloway GQ. Laparoscopic Collis gastroplasty is the treatment of choice for the shortened esophagus. Am J Surg 1996;171:477-81. [Crossref] [PubMed]
- Pearson FG, Cooper JD, Patterson GA, et al. Gastroplasty and fundoplication for complex reflux problems. Long-term results. Ann Surg 1987;206:473-81. [Crossref] [PubMed]
- Bochkarev V, Lee YK, Vitamvas M, et al. Short esophagus: how much length can we get? Surg Endosc 2008;22:2123-7. [Crossref] [PubMed]
- Madan AK, Frantzides CT, Patsavas KL. The myth of the short esophagus. Surg Endosc 2004;18:31-4. [Crossref] [PubMed]
- Dallemagne B, Kohnen L, Perretta S, et al. Laparoscopic repair of paraesophageal hernia. Long-term follow-up reveals good clinical outcome despite high radiological recurrence rate. Ann Surg 2011;253:291-6. [Crossref] [PubMed]
- Terry ML, Vernon A, Hunter JG. Stapled-wedge Collis gastroplasty for the shortened esophagus. Am J Surg 2004;188:195-9. [Crossref] [PubMed]
- Mattioli S, Lugaresi ML, Costantini M, et al. The short esophagus: intraoperative assessment of esophageal length. J Thorac Cardiovasc Surg 2008;136:834-41. [Crossref] [PubMed]
- Morino M, Giaccone C, Pellegrino L, et al. Laparoscopic management of giant hiatal hernia: factors influencing long-term outcome. Surg Endosc 2006;20:1011-6. [Crossref] [PubMed]
- Zehetner J, DeMeester SR, Ayazi S, et al. Laparoscopic wedge fundectomy for collis gastroplasty creation in patients with a foreshortened esophagus. Ann Surg 2014;260:1030-3. [Crossref] [PubMed]
- Awais O, Luketich JD, Schuchert MJ, et al. Reoperative antireflux surgery for failed fundoplication: an analysis of outcomes in 275 patients. Ann Thorac Surg 2011;92:1083-9; discussion 1089-90. [Crossref] [PubMed]
- Horvath KD, Swanstrom LL, Jobe BA. The short esophagus: pathophysiology, incidence, presentation, and treatment in the era of laparoscopic antireflux surgery. Ann Surg 2000;232:630-40. [Crossref] [PubMed]
- Urbach DR, Khajanchee YS, Glasgow RE, et al. Preoperative determinants of an esophageal lengthening procedure in laparoscopic antireflux surgery. Surg Endosc 2001;15:1408-12. [Crossref] [PubMed]
- Yano F, Stadlhuber RJ, Tsuboi K, et al. Preoperative predictability of the short esophagus: endoscopic criteria. Surg Endosc 2009;23:1308-12. [Crossref] [PubMed]
- Awad ZT, Filipi CJ, Mittal SK, et al. Left side thoracoscopically assisted gastroplasty: a new technique for managing the shortened esophagus. Surg Endosc 2000;14:508-12. [Crossref] [PubMed]
- Johnson AB, Oddsdottir M, Hunter JG. Laparoscopic Collis gastroplasty and Nissen fundoplication. A new technique for the management of esophageal foreshortening. Surg Endosc 1998;12:1055-60. [Crossref] [PubMed]
- Swanstrom LL, Sopher NJ. Mastery of Laparoscopic and Endoscopic Surgery, 4th edition. Philadelphia: Lippincott Williams & Wilkins, 2015
- Lugaresi M, Mattioli B, Perrone O, et al. Results of left thoracoscopic Collis gastroplasty with laparoscopic Nissen fundoplication for the surgical treatment of true short oesophagus in gastro-oesophageal reflux disease and Type III-IV hiatal hernia. Eur J Cardiothorac Surg 2016;49:e22-30. [Crossref] [PubMed]
- Youssef YK, Shekar N, Lutfi R, et al. Long-term evaluation of patient satisfaction and reflux symptoms after laparoscopic fundoplication with Collis gastroplasty. Surg Endosc 2006;20:1702-5. [Crossref] [PubMed]
- Lin E, Swafford V, Chadalavada R, et al. Disparity between symptomatic and physiologic outcomes following esophageal lengthening procedures for antireflux surgery. J Gastrointest Surg 2004;8:31-9; discussion 38-9. [Crossref] [PubMed]
- Jobe BA, Horvath KD, Swanstrom LL. Postoperative function following laparoscopic collis gastroplasty for shortened esophagus. Arch Surg 1998;133:867-74. [Crossref] [PubMed]
Cite this article as: Riva P, Swanström LL. Collis gastroplasty: why, when and how? Ann Esophagus 2018;1:12.


