
Concurrent radiofrequency ablation and Nissen fundoplication
Barrett’s esophagus (BE) remains filed of great controversies, starting from its definition, over the course of disease evolution, till the issue of treatment. Still, somehow it seems that with the introduction of Barrx (Medtronic) radiofrequency ablation (RFA) system things change towards better, as we gained effective weapon to safely and efficiently treat BE. Although showed to be exceptionally effective, Barrx RFA is still relatively a new method, and years must pass, before definitive judgment is generated. The evidences and results that emerged so far address two important issues, possible points in which improvement of the RFA procedure may be conceivable.
These issues are recurrence of BE after successful and complete eradication (CE) of BE with RFA, and technical difficulties for the proper procedure accomplishment in case of significant hiatal hernia with dilated distal esophagus. Of course, one cannot completely divide these two aspects, as by experience of the authors of this article, significant hiatal hernia together with long segment of BE represent a major contributing factor in BE recurrence.
According to the results of the 5-year follow-up of patients previously included in AIM dysplasia trial the incidence rate of BE recurrence was 10.8 per 100 person-year (1). The recurrence of the BE was most common in first year after RFA, and much less common in 4th and 5th year after ablation. Here we can draw a conclusion that this early onset of recurrence might be related to: (I) incomplete ablation in first place, (II) ongoing reflux, (III) inability of adequate reflux control with standard PPI protocol after ablation.
Possible causes of recurrence are well analyzed in paper from Krishnan et al. (2). This study included 37 patients with long segment BE IM, and patients with HGD. After the 3 consecutive ablation sessions, patients were divided in the groups with complete response (CR) and incomplete response (ICR). 24-hour pH/impedance data showed that those with ICR had significantly higher number of weakly acid and weakly alkaline reflux opposed to those with CR. Since all patients were given PPI’s in a twice-daily regimen, the number of acid reflux episodes did not differ between the groups. Same study recognized the size of hiatal hernia and length of the BE to be independent predictive factors for incomplete response after RFA. Study by Akiyama et al. was in concordance with these data (3). It was shown that patients with normal or mild intraesophageal acid exposure have significantly higher probability of achieving CR after RFA opposed to those with moderate and severe esophageal acid exposure, measured with 24-hour pH metry. Along with the acid exposure, size of hiatal hernia was recognized also as an independent risk factor for RFA failure. Some other studies have identified size of hiatal hernia and length of the BE as the most important factors for successful RFA procedure (4).
When analyzing issue of recurrence, we have to go beyond adopted circle of thoughts and deeds. According to concept of Chandrasoma et al., true extent of BE is under recognized in daily clinical practice (5). As the process of intestinilization of esophagus flows, distal part of the esophagus looses its tubular shape, getting the macroscopic appearance of proximal stomach. In a majority of patients with long extent of BE, which are candidates for RFA, this distal esophagus is so severely damaged, together with complete loss of distal esophageal sphincter, and anatomical dilatation and deviation of distal segment. This makes proper Barrx 360 ablation almost impossible in some cases, and might be a reason behind several attempts for achieving complete BE eradication and early recurrence (Figure 1). This also may be the cause, why in most instances, recurrence of BE is found at the level of esophagogastric junction (6,7).
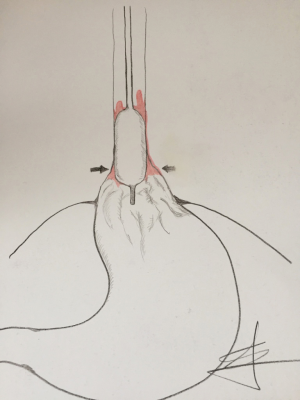
Having this in mind, we adopted the concept of concurrent antireflux surgery and RFA. Goers et al. first described this approach in the literature (8). Their study was conducted in 8 patients, of which 6 was presented with major hiatal hernia requiring reduction. The procedure was concomitant HALO 360 RFA during the laparoscopic fundoplication. RFA was performed after hernia reduction, and esophageal encirclement, which affected the esophageal lumen in the manner that RFA electrode applied more closely to the mucosa. This kind of approach showed that in 5 patients CE of BE was achieved after a single RFA session, while the remaining three patients underwent consequent ablation session. The rates of procedure related complications were substantially high. One patient in this small study group developed stricture, and in one esophageal perforation occurred. The cause of perforation may be related to the fact, that in this particular patient BE segment was too long to be treated in a single session.
In our study, concomitant LNF and RFA procedure was proven to be safe (9). Procedure was not too time consuming, and it was performed in conjunction with the basic principles of Barrx 360 procedure. Most importantly the esophageal calibration was performed after the hiatal hernia had been reduced, esophagus encircled and pulled down. That way the potential pitfall of choosing wrong diameter of RFA balloon was avoided. During this step physician can clearly endoscopically visualize how distal esophageal widening or deviation of the lumen is lost. On the other hand, surgeon can be completely sure that the balloon RFA electrode is placed in a right position in the distal esophagus (Figure 2). Only by this way, one can truly understand the true esophagogastric junction anatomy, and to observe that what we interpret in the beginning of the endoscopy procedure as gastric mucosa, is instead distal segment of the esophagus, intestinilized and widened.
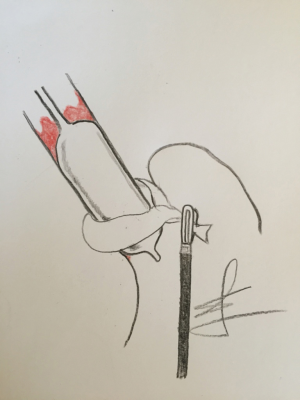
No more than 6 cm of BE mucosa was treated in the single session, according to the procedure guidelines. There was no procedure related complications in our study.
Another interesting observation, which we couldn’t document so far, is that patient with concomitant or prior fundoplication, have faster mucosal recovery after RFA, observed on the first endoscopic evaluation. It has been noticed in our study that patients in whom PPI’s are employed in a twice-daily regimen after RFA, have very fragile mucosa 8 weeks after procedure, when first endoscopy is usually scheduled. This mucosa is sometimes covered with superficial erosions. In this subset of patients we usually add evening dose of H2 blocker, along with previous PPI therapy. The higher number of patients is needed to document these observations, and more standardization in the term of interobserver agreement. This is why we initiated the study in which we will compare effects of Nissen fundoplication concurrent with RFA and after RFA, with the final goal to evaluate the effect of this approach on early BE recurrence.
To conclude, antireflux surgery may have important role in some patients undergoing RFA, especially those with severe anatomical impairment in distal esophageal segment. As a concurrent procedure, it may be beneficial in the terms of reducing the early recurrence rates, which seems to be important issue in RFA. By doing synchronous RFA and hiatal hernia repair and fundoplication, one might observe a true anatomy of esophagogastric junction in its entirety, and might be able to truly observe the distal extent of columnar esophagus.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Luigi Bonavina) for the series “Gastroesophageal Reflux Disease” published in Annals of Esophagus. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoe.2018.08.03). The series “Gastroesophageal Reflux Disease” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cotton CC, Wolf A, Overholt B, et al. Late recurrence of intestinal metaplasia after complete eradication of intestinal metaplasia is rare: Final report from Ablation of intestinal metaplasia containing dysplasia trial. Gastroenterol 2017;153:681-8.e2. [Crossref] [PubMed]
- Krishnan K, Pandolfino J, Kahrilas P, et al. Increased Risk for Persistent Intestinal Metaplasia in Patients with Barrett’s Esophagus and Uncontrolled Reflux Exposure before Radiofrequency Ablation. Gastroenterol 2012;143:576-81. [Crossref] [PubMed]
- Akiyama J, Marcus SN, Triadafilopoulos G. Effective intra-esophageal acid control is associated with improved radiofrequency ablation outcomes in Barrett's esophagus. Dig Dis Sci 2012;57:2625-32. [Crossref] [PubMed]
- Korst RJ, Santana-Joseph S, Rutledge JR, et al. Effect of hiatal hernia size and columnar segment length on the success of radiofrequency ablation for Barrett's esophagus: a single-center, phase II clinical trial. J Thorac Cardiovasc Surg 2011;142:1168-73. [Crossref] [PubMed]
- Chandrasoma P, DeMeester T. New histological definitions of the esophagus, stomach and esophagogastric junction. In: GERD: from reflux to adenocarcinoma. Amsterdam; Boston: Elsevier/AP, pp 135-47.
- Vaccaro BJ, Gonzalez S, Poneros JM, et al. Detection of intestinal metaplasia after successful eradication of Barrett’s Esophagus with radiofrequency ablation. Dig Dis Sci 2011;56:1996-2000. [Crossref] [PubMed]
- Korst RJ, Santana-Joseph S, Rutledge JR, et al. Patterns of recurrent and persistent intestinal metaplasia after successful radiofrequency ablation of Barrett's esophagus. J Thorac Cardiovasc Surg 2013;145:1529-34. [Crossref] [PubMed]
- Goers TA, Leão P, Cassera MA, et al. Concomitant endoscopic radiofrequency ablation and laparoscopic reflux operative results in more effective and efficient treatment of Barrett esophagus. J Am Coll Surg 2011;213:486-92. [Crossref] [PubMed]
- Skrobić O, Simić A, Radovanović N, et al. Significance of Nissen fundoplication after endoscopic radiofrequency ablation of Barrett's esophagus. Surg Endosc 2016;30:3802-7. [Crossref] [PubMed]
Cite this article as: Simić AP, Skrobić OM, Ivanović N, Peško PM. Concurrent radiofrequency ablation and Nissen fundoplication. Ann Esophagus 2018;1:10.


