
Remedial bypass or antrectomy and Roux-en-Y after failed fundoplication
Introduction
Gastroesophageal reflux disease (GERD) has reached endemic proportions in Western countries, and up to 20% of adults with GERD experience symptoms on a weekly basis (1). Although acid suppressive therapy is ubiquitously used for management of symptoms, laparoscopic fundoplication is the gold standard for definitive treatment of GERD (2). Excellent long-term results have been reported from high-volume centers, with more than 90% patient satisfaction at 5, 10, and even 20 years (3,4). However, some patients report recurrent symptoms or develop undesirable new symptoms, which may require reoperation (5). Reoperation, most commonly in the form of redo fundoplication, is associated with increased perioperative morbidity and mortality, along with worse patient-reported outcomes (6). Some patients undergoing reoperation are either poor candidates for redo fundoplication or cannot undergo redo fundoplication due to iatrogenic damage of the gastroesophageal junction (GEJ) or fundus. These patients are better candidates for either a Roux-en-Y (RNY) reconstruction or esophageal resection (5,7-9).
In the modern laparoscopic era, the RNY has become the sine qua non with bariatric procedures, but RNY reconstruction has long been used as an antireflux procedure, especially for complex diseases or after failed fundoplication. Roux-en-Y reconstruction with gastrojejunal anastomosis to a proximal gastric pouch (with or without distal gastrectomy) is in itself an excellent anti-GERD measure, as it diverts bile and reflux while removing the refluxate reservoir (i.e., it breaks the continuity of the stomach and the esophagus). One could argue the relative merits of RNY, but in patients with GERD, there is no physiological difference between RNY reconstruction with the distal stomach left in situ (i.e., bypass) or with the distal stomach resected (i.e., distal gastrectomy).
Historical perspectives
In early attempts at gastrointestinal tract resection and reconstruction, surgeons strove to maintain the “normal” sequence of organs—that is, the esophagus to the stomach to the duodenum. However, diversion from this normal anatomical sequence for disease control was subsequently described. The Billroth II procedure was the name given to a reconstruction technique after antrectomy; it involved use of a loop gastrojejunostomy (10). The classic en-Y reconstruction was described by Caesar Roux in 1893 (11). The RNY procedure has several applications in gastrointestinal surgery, and complements the Billroth II procedure by avoiding bile reflux and its consequences.
The role of RNY gastrojejunostomy as an antireflux measure predates anti-secretory therapy. In 1956, Ellis used an antrectomy with RNY gastrojejunostomy in a surgically induced reflux disease model (i.e., cardiotomy with esophagogastrostomy), and showed that it was a viable treatment option for complex reflux esophagitis (12). In 1984, Washer et al. published the results of a randomized trial that compared Nissen fundoplication and antrectomy with RNY. Washer et al. showed that the latter was a better option for irreducible hiatal hernia and for reoperative cases. Ellis later published his 34 years of clinical experience with RNY for complex GERD patients who required reoperation, and described a respectable success rate (13). Csendes et al. published several summaries of his continuing work using bypass as an antireflux measure, both in patients with normal weight and in obese patients. In 2002, he reported long-term follow-up of 210 “vagotomy-partial gastrectomy with duodenal diversion” procedures in patients with Barrett’s esophagus, and reported remarkable outcomes (14). Advancing his work, Braghetto et al. reported the results of his prospective comparison of three procedures for reflux in obese patients: calibrated fundoplication with posterior gastropexy; fundoplication with vagotomy, distal gastrectomy, and RNY gastrojejunostomy; and laparoscopic resectional RNY gastric bypass. Braghetto et al. found superior outcomes in both bypass groups, and a significant difference in weight loss results (15).
Fundoplication with distal gastrectomy or subtotal gastrectomy without fundoplication are acceptable options for definitive treatment of complex GERD. The former is more useful in patients with severe delayed gastric emptying, and the latter is more efficacious in patients for whom a good fundoplication is not feasible (e.g., those with a damaged fundus or extremely poor motility, especially with short or aperistaltic esophagus).
Widespread application of RNY gastric bypass as a bariatric procedure revealed its secondary effect on amelioration of GERD (16). In RNY gastric bypass procedures, the distal stomach is left in situ. This paved the way for RNY gastrojejunostomy without distal gastrectomy as a viable option for addressing reflux in both primary and reoperative procedures. Reconstruction without distal gastrectomy allows surgeons to preserve the stomach for potential use as a conduit in the event of future esophagectomy for worsening esophageal motility or for progression of Barrett’s esophagus. This also obviates the risk of the dreaded duodenal stump leak, which can occur in patients who have undergone gastrectomy.
Risk factors associated with redo fundoplication
Several large series and meta-analyses have shown that redo fundoplication is associated with significantly worse patient-reported outcomes than primary anti-reflux surgery (6,17). Redo fundoplication also carries greater perioperative morbidity and mortality (18). The subsequent sections detail factors that may contribute to worse outcomes after redo fundoplication.
Obesity
Patients with an elevated body mass index (BMI) also have higher intraabdominal pressure, which puts stress on the crus closure and fundoplication sutures. Morbid obesity (i.e., BMI >35 kg/m2) has reportedly been associated with poorer outcomes after primary and redo fundoplication; however, RNY with gastric bypass is often carried out in morbidly obese patients due to its secondary benefits of weight loss and its tendency to be reimbursed by health insurance providers. Interestingly, several papers have shown equivalent outcomes between patients with higher BMI (up to 40 kg/m2) and patients with BMI within the reference range (19,20).
Akimoto et al. previously reported that patterns of failure after fundoplication were associated with BMI (21), and found similar patterns of failure for patients with BMI 30–35 kg/m2 and patients with BMI 35 kg/m2. These patterns were distinct from patients with BMI <30 kg/m2, indicating that similar physiological forces were at play at BMI >30 kg/m2 rather than >35 kg/m2. In a separate retrospective analysis, Olson et al. graphed patient-reported outcomes along a linear BMI scale rather than comparing categorical groups. Patients with BMI >32 kg/m2 had significantly worse outcomes after redo fundoplication. Regardless of absolute value, increasing BMI seems to be associated with poorer outcomes after redo fundoplication; therefore, an alternative antireflux procedure (e.g., RNY reconstruction) should be considered for obese patients (22).
Multiple previous fundoplication
As mentioned above, redo fundoplication is associated with higher morbidity and mortality, along with poorer patient-reported outcomes. Negative outcomes of redo fundoplication may include greater prevalence of short esophagus, esophageal dysmotility, delayed gastric emptying compounded by increased inherent risk of vagal nerve injury, and damage to the fundus. The likelihood of these undesirable conditions increases with each reoperation. When a patient has undergone 3 or more fundoplications, they may be at increased risk for vagal injury and poor outcomes (23,24). We also found similar results (25). It is probably prudent to consider RNY reconstruction as the preferred option for the 2nd reoperation, especially if the initial procedure was performed at a center of expertise.
Short esophagus
A successful fundoplication mandates partial or complete wrap of the fundus around the distal esophagus, which must lie tension-free below the diaphragm. This requires 2 to 3 cm of intraabdominal esophageal length; absence of this length after esophageal mobilization is called short esophagus. Collis gastroplasty with fundoplication is the preferred procedure for a patient with a short esophagus. Perhaps no other topic in foregut surgery evokes a more visceral argument than the enigmatic short esophagus; in fact, some experts deny its very existence. However, most surgeons agree that short esophagus not only exists, but it also must be addressed for successful outcomes after fundoplication.
Short esophagus is more prevalent in reoperations, implying a missed diagnosis at the time of the previous procedure. Short esophagus results in undue tension and, ultimately, failure of the fundoplication. However, Collis gastroplasty with fundoplication has less-than-ideal outcomes. This is especially the case in patients with esophageal dysmotility (26). In our opinion, in the reoperative setting, an RNY reconstruction should be strongly considered if a short esophagus is identified, especially if preoperative symptoms included dysphagia or if preoperative testing showed ineffective esophageal motility. Additionally, if a previous fundoplication involved a Collis procedure, a redo fundoplication is likely already doomed to have poor outcomes, and an RNY conversion is the most viable option.
Esophageal dysmotility
Severe esophageal dysmotility with recalcitrant GERD is noted in a subset of patients, especially those with scleroderma. It has been reported that an RNY reconstruction is a better option than partial fundoplication; RNY also carries lower morbidity than an esophagectomy for control of reflux in scleroderma patients (27). There is a higher prevalence of moderate or severe dysmotility in these patients, especially those with a short esophagus or those who have undergone more than one previous fundoplication. Such patients should instead be considered for RNY reconstruction. Additionally, because dysmotility may worsen over time and ultimately require an esophagectomy, it is imperative that the distal stomach be left in situ.
Damaged GEJ or fundus
Reoperation requires complete takedown of the previous fundoplication and distal esophageal mobilization before either redo fundoplication or RNY conversion. Not infrequently, patients undergoing reoperation have dense adhesions, and the takedown portion of the procedure results in full thickness or seromuscular damage of the GEJ, the fundus, or both. Although smaller defects (especially in the fundus) can be managed with primary repair, larger defects preclude proper fundoplication. In this situation, it is preferable to proceed with RNY, even if the RNY requires an esophagojejunal anastomosis. The use of mesh, including absorbable biosynthetic mesh, in earlier procedures increases the risk of damage to the GEJ and/or the fundus during takedown; in fact, there is a high need for conversion to RNY in such cases (28).
Delayed gastric emptying
In most patients with delayed gastric emptying, RNY conversion for another reason (such as one of those listed above) would eliminate the need for gastrectomy. Patients who experience delayed gastric emptying—especially if the condition is persistent after previous pyloroplasty—are patients best managed with RNY conversion. In patients who experience delayed gastric emptying but exhibit none of the other factors listed above, redo fundoplication with distal gastrectomy is an excellent choice for good outcomes.
How we do RNY conversion
Reoperative antireflux surgery with RNY conversion requires 3 distinct steps: (I) takedown of previous fundoplication, (II) restoration of the intraabdominal length of esophagus and crus closure, and (III) RNY reconstruction, with or without distal gastrectomy. After complete takedown of the previous fundoplication and mobilization of the esophagus, the hiatus is closed and an intraoperative esophagogastroduodenoscopy (EGD) is performed to assess complete takedown and to rule out injury to the GEJ or the fundus. If RNY conversion is the next step, there is no need for a Collis gastroplasty (in patients with a short esophagus). Additionally, mesh reinforcement of the hiatus is not as critical. Whether to proceed via laparotomy or laparoscopy is a matter of surgeon preference. For surgeons with sufficient experience, many cases can be performed laparoscopically, which has the benefits of decreased perioperative pain and shorter hospital stay. In rare cases, we have had to perform a left-sided transthoracic mobilization of a herniated stomach (the patient had a healed gastric perforation of a deep penetrating ulcer near vascular structures), followed by transabdominal RNY conversion.
An RNY conversion includes 3 critical steps: (I) selecting esophagojejunal vs. gastrojejunal anastomosis, including the size of the proximal gastric pouch; (II) determining the length of biliary and alimentary limbs of the RNY; and (III) assessing the state of the distal stomach and choosing whether to resect it or to leave it in situ. See Figure 1 for a schematic summary of procedural approaches.
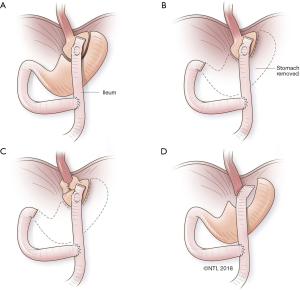
Selecting esophagojejunal vs. gastrojejunal anastomosis, including the size of the proximal gastric pouch
In patients with extensive damage to the GEJ from takedown or from recalcitrant stricture, there is no alternative but to proceed with esophagojejunal anastomosis. We recommend a proximal gastric pouch along the lesser curvature rather than transversely across the fundus. In traditional RNY for bariatric procedures, the proximal pouch is very small (i.e., 15 to 30 cc). However, it may be wiser to make a larger pouch in reoperations, due to potential devascularization of the proximal-most stomach below the GEJ during takedown. In addition, such a small pouch has been associated with postoperative dysphagia.
We assess the vascularization of the lesser curvature and go at least 4 to 5 cm below that to start our pouch construction. At times, this may be even at the incisura angularis, if takedown of a slipped fundoplication has devascularized several centimeters of proximal lesser curvature. A defect is made in the lesser mesentery, next to the chosen spot on the lesser curvature. A linear stapler is fired (45 mm) perpendicular to the lesser curvature. The serial loads of staplers are fired parallel to the lesser curvature, up to the angle of His. Care is taken to follow the arc of the lesser curvature, so as not to make the proximal-most section too narrow. The gastric pouch holds somewhere between 60 and 100 cc, allowing the patient to have a meal of a reasonable size. An EGD is carried out to check for air leaks in the pouch.
Determining the length of biliary and alimentary limbs of the RNY
For traditional bariatric RNY gastric bypass, a biliary limb of 60 cm and an alimentary limb between 100 and 150 cm are used. To prevent bile reflux, a minimum length of 40 cm for the alimentary limb has been proposed, and with a margin of error, a 60-cm alimentary limb is most commonly used, even in malignant gastric resections. The length of the biliary limb does not affect bile reflux. We identify the ligament of Treitz and run the bowel distally to choose the first section, which will easily reach the location of the proximal anastomosis. This is generally about 15 to 20 cm long. The bowel is divided with a 60-cm linear vascular stapler. The distal bowel is run for the desired alimentary length (a minimum of 60 cm), and an RNY anastomosis is created at that level with the end of the biliary limb. The alimentary limb is brought in an anti-colic fashion to lie next to the proximal gastric pouch/esophagus for anastomosis. After anastomosis, a repeat EGD is performed to check for leaks, and a nasogastric tube is placed beyond the anastomosis.
Assessing the state of the distal stomach and choosing whether to resect it or leave it in situ
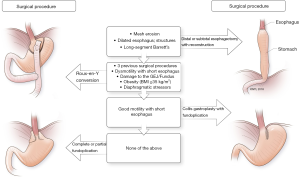
We leave the distal stomach in situ whenever possible to help decrease operative time and to lessen morbidity of distal gastrectomy, including the dreaded duodenal stump leak. The distal stomach can also be used for reconstruction if an esophagectomy is needed in the future, in case of malignant deterioration from Barrett’s esophagus or progression of esophageal dysmotility. Additionally, the gastric remnant can be used for a perioperative gastrostomy tube for delivery of medications and nutrition in these often-complex and debilitated patients. Except in extreme circumstances (e.g., recalcitrant ulcers or peptic stricture of the distal stomach), there is no medical reason for distal gastrectomy. The greater curvature is occasionally so devascularized that the distal stomach is not amenable for use as future gastric conduit; in such situations, it may be prudent to proceed with gastrectomy. Figure 2 summarizes our decision-making process on selecting the tailored procedure for the patient.
Postoperative care
After an RNY procedure, patients are managed in the same fashion as after any foregut surgery. We routinely do a radiographic swallow study on postoperative day 1 (or later, for patients who have undergone an open procedure) with water-soluble contrast. If no leak is identified, a bariatric liquid diet is initiated. We usually discharge the patient home on a liquid diet (with instructions on how to avoid foods with high sugar content) and ulcer prophylaxis (i.e., famotidine 20 mg twice daily) after passing flatus.
Patients are advanced to a post-gastrectomy diet over the next 2 to 3 weeks. We also recommend that patients take a multivitamin each day and check the nutritional panel at 6 months after surgery as a precaution, even though we have yet to identify new nutritional deficiencies (other than any preexisting deficiencies). Occasionally, doses of medications generally absorbed in the duodenum or proximal small bowel (e.g., levothyroxine) need to be adjusted over time. In the long term, both the patient and the physician must be aware of closed-loop obstruction due to internal hernia.
Outcomes of RNY conversion for reoperative antireflux surgery
Few studies report exclusively on outcomes of RNY conversion as a reoperative intervention after previous antireflux surgery. Ellis et al. reported a small series of 33 patients; these researchers used an open approach and had an acceptable 27% morbidity rate as well as excellent symptom control (72% of patients) at a mean follow-up of 534 months (29). Awais et al. reported their outcomes of RNY conversion in 105 patients for whom antireflux operations had failed, describing a high number of previous Collis fundoplications (i.e., 26%). In this series, 46% of patients had 2 or more previous procedures; still, Awais et al. were able to complete 54% of the cases laparoscopically, with a major postoperative complication rate of 21% (9).
Makris et al. reported a series of 72 patients who underwent RNY conversion, with up to 5-year follow-up. They reported significant improvement in heartburn, regurgitation, and dysphagia scores after the procedure. In their series, a subset of patients who were underweight before surgery actually gained weight after resolution of symptoms, whereas morbidly obese patients lost some weight (6). Mittal et al. previously reported a large series of 130 patients who underwent redo fundoplication and RNY conversion, and found equivalent symptom control and patient satisfaction in both groups, despite a significantly higher prevalence of risk factors for poor outcomes in the RNY group compared to the redo fundoplication group. Patients who underwent RNY had better outcomes than patients who underwent redo fundoplication for the 3rd time or more (8). Juhasz et al. reported better comes with RNY conversion than with redo fundoplication in patients undergoing reoperative procedures for a recurrent hiatal hernia measuring more than 5 cm. They attributed this to a higher possibility of short esophagus in this subgroup of patients (30). Singhal et al. reported a large series of primary and reoperative procedures, and described a significantly higher use of RNY conversion as the preferred reoperative strategy in patients undergoing their 2nd or more reoperation (18).
Conclusions
Overall, several factors are taken into account for reoperative antireflux surgery or when considering RNY conversion. We think of RNY as but one tool for amelioration of symptoms in patients who have undergone previous antireflux procedures. In some patients with mesh erosion, long-segment Barrett’s esophagus (and young age), profound esophageal dysmotility with a dilated esophagus, and strictures that cannot be dilated, the only surgical option is a distal or subtotal esophagectomy with reconstruction.
Our operative planning and procedure loosely follow the diagram in the Figure2. Overall, several options for reoperative interventions exist, and are tailored to the patient’s symptoms, underlying physiology, and intraoperative findings. We believe that RNY conversion is a better alternative to redo fundoplication in patients who require reoperation, or for those who have a short esophagus (especially with poor motility); profound esophageal dysmotility; delayed gastric emptying; BMI >32 kg/m2 (definitely for patients with BMI >35 kg/m2); or large, recurrent hiatal hernia. RNY conversion is the only option when the GEJ or fundus has been damaged intraoperatively.
Acknowledgments
The authors wish to thank Clare Sonntag for her editorial assistance.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Luigi Bonavina) for the series “Gastroesophageal Reflux Disease” published in Annals of Esophagus. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoe.2018.08.02). The series “Gastroesophageal Reflux Disease” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- El-Serag HB, Petersen NJ, Carter J, et al. Gastroesophageal reflux among different racial groups in the United States. Gastroenterology 2004;126:1692-9. [Crossref] [PubMed]
- Rickenbacher N, Kötter T, Kochen MM, et al. Fundoplication versus medical management of gastroesophageal reflux disease: systematic review and meta-analysis. Surg Endosc 2014;28:143-55. [Crossref] [PubMed]
- Draaisma WA, Rijnhart-de Jong HG, Broeders IA, et al. Five-year subjective and objective results of laparoscopic and conventional Nissen fundoplication: a randomized trial. Ann Surg 2006;244:34-41. [Crossref] [PubMed]
- Granderath FA, Kamolz T, Schweiger UM, et al. Long-term results of laparoscopic antireflux surgery. Surg Endosc 2002;16:753-7. [Crossref] [PubMed]
- Awais O, Luketich JD, Schuchert MJ, et al. Reoperative Antireflux Surgery for Failed Fundoplication: An Analysis of Outcomes in 275 Patients. Ann Thorac Surg 2011;92:1083-9; discussion 1089-90. [Crossref] [PubMed]
- Furnée EJB, Draaisma WA, Broeders IA, et al. Surgical reintervention after failed antireflux surgery: a systematic review of the literature. J Gastrointest Surg 2009;13:1539-49. [Crossref] [PubMed]
- Makris KI, Panwar A, Willer BL, et al. The role of short-limb Roux-en-Y reconstruction for failed antireflux surgery: a single-center 5-year experience. Surg Endosc 2012;26:1279-86. [Crossref] [PubMed]
- Mittal SK, Légner A, Tsuboi K, et al. Roux-en-Y reconstruction is superior to redo fundoplication in a subset of patients with failed antireflux surgery. Surg Endosc 2013;27:927-35. [Crossref] [PubMed]
- Awais O, Luketich JD, Reddy N, et al. Roux-en-Y Near Esophagojejunostomy for Failed Antireflux Operations: Outcomes in More Than 100 Patients. Ann Thorac Surg 2014;98:1905-11; discussion 1911-3.
- von Hacker VR. Zur casuistik und statistik der magenresectionen und gastroenterostomieen. Verhandlungen der Deutschen Gesellschaft fur Chirurgie 1885;14:62-71.
- Roux C. De la gastroenterostomie. In: Revue de chirurgie, 1893:402-3.
- Ellis FH. Experimental Aspects of the Surgical Treatment of Reflux Esophagitis and Esophageal Stricture. Ann Surg 1956;143:465-70. [Crossref] [PubMed]
- Washer GF, Gear MW, Dowling BL, et al. Randomized prospective trial of Roux-en-Y duodenal diversion versus fundoplication for severe reflux oesophagitis. Br J Surg 1984;71:181-4. [Crossref] [PubMed]
- Csendes A, Burdiles P, Braghetto I, et al. Early and late results of the acid suppression and duodenal diversion operation in patients with Barrett’s esophagus: analysis of 210 cases. World J Surg 2002;26:566-76. [Crossref] [PubMed]
- Braghetto I, Korn O, Csendes A, et al. Laparoscopic treatment of obese patients with gastroesophageal reflux disease and Barrett’s esophagus: a prospective study. Obes Surg 2012;22:764-72. [Crossref] [PubMed]
- Valezi AC, Herbella FAM, Schlottmann F, Patti MG. Gastroesophageal Reflux Disease in Obese Patients. J Laparoendosc Adv Surg Tech A 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Little AG, Ferguson MK, Skinner DB. Reoperation for failed antireflux operations. J Thorac Cardiovasc Surg 1986;91:511-7. [PubMed]
- Singhal S, Kirkpatrick DR, Masuda T, et al. Primary and Redo Antireflux Surgery: Outcomes and Lessons Learned. J Gastrointest Surg 2018;22:177-86. [Crossref] [PubMed]
- Luketina RR, Koch OO, Köhler G, et al. Obesity does not affect the outcome of laparoscopic antireflux surgery. Surg Endosc 2015;29:1327-33. [Crossref] [PubMed]
- Martin Del Campo SE, Chaudhry UI, Kanji A, et al. Laparoscopic Nissen fundoplication controls reflux symptoms and improves disease-specific quality of life in patients with class I and II obesity. Surgery 2017;162:1048-54. [Crossref] [PubMed]
- Akimoto S, Nandipati KC, Kapoor H, et al. Association of Body Mass Index (BMI) with Patterns of Fundoplication Failure: Insights Gained. J Gastrointest Surg 2015;19:1943-8. [Crossref] [PubMed]
- Olson MT, Singhal S, Panchanathan R, et al. Primary paraesophageal hernia repair with Gore® Bio-A® tissue reinforcement: long-term outcomes and association of BMI and recurrence. Surg Endosc 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Ellisjr F, Gibb S, Heatley G. Reoperation after failed antireflex surgery*Review of 101 cases. Eur J Cardiothorac Surg 1996;10:225-31; discussion 231-2. [Crossref] [PubMed]
- Papasavas PK, Yeaney WW, Landreneau RJ, et al. Reoperative laparoscopic fundoplication for the treatment of failed fundoplication. J Thorac Cardiovasc Surg 2004;128:509-16. [Crossref] [PubMed]
- Légner A, Tsuboi K, Bathla L, et al. Reoperative antireflux surgery for dysphagia. Surg Endosc 2011;25:1160-7. [Crossref] [PubMed]
- Bathla L, Legner A, Tsuboi K, et al. Efficacy and feasibility of laparoscopic redo fundoplication. World J Surg 2011;35:2445-53. [Crossref] [PubMed]
- Kent MS, Luketich JD, Irshad K, et al. Comparison of surgical approaches to recalcitrant gastroesophageal reflux disease in the patient with scleroderma. Ann Thorac Surg 2007;84:1710-5; discussion 1715-6.
- Stadlhuber RJ, Sherif AE, Mittal SK, et al. Mesh complications after prosthetic reinforcement of hiatal closure: a 28-case series. Surg Endosc 2009;23:1219-26. [Crossref] [PubMed]
- Ellis FH, Gibb SP. Vagotomy, antrectomy, and Roux-en-Y diversion for complex reoperative gastroesophageal reflux disease. Ann Surg 1994;220:536-42; discussion 542-3. [Crossref] [PubMed]
- Juhasz A, Sundaram A, Hoshino M, et al. Outcomes of surgical management of symptomatic large recurrent hiatus hernia. Surg Endosc 2012;26:1501-8. [Crossref] [PubMed]
Cite this article as: Kovacs B, Mittal SK. Remedial bypass or antrectomy and Roux-en-Y after failed fundoplication. Ann Esophagus 2018;1:8.


