
Bile acids (taurocholic acid, taurodeoxycholic acid, taurochenodeoxycholic acid, tauroursodeoxycholic acid) develop esophageal cancer in a rat model of duodenoesophageal anastomosis after total gastrectomy
Introduction
Gastrectomy is the most common gastrointestinal surgery in Japan. Thoracic esophageal cancer in patients who underwent surgery has a history of gastrectomy in 3–10% of cases (1). Whether post-gastrectomy conditions lead to esophageal carcinogenesis is controversial. Distal gastrectomy is a good model for clinically examining esophageal reflux. It has been reported that symptomatic duodenal gastric reflux is the most frequent symptom of post-gastric surgery and is present in 35% (2).
Gastrectomy causes truncal vagotomy (TV), widening of the angle of His and small gastric remnant reduction. TV causes a decrease in gastric motility. The widening of angle of His causes deterioration of lower esophageal sphincter (LES). A small remnant stomach cannot accommodate a large amount of stomach content. These factors facilitate reflux of duodenal juice into the esophagus after gastrectomy.
We retrospectively evaluated 153 patients (3) undergoing subtotal esophagectomy for thoracic esophageal cancer. Divided into two groups, gastrectomy group, non-gastrectomy group. Group 1: 14 patients undergoing gastrectomy and Group 2: 139 patients not undergoing gastrectomy. The esophageal cancer occupies most of the lower esophagus in the post-gastric resection group, while the non-gastric resection group often has the middle esophagus. The histologic subtype was squamous cell carcinoma (SCC) in both gastric resection group and non-gastric resection group. Changes in the esophageal mucosa due to persistent reflux of stomach and duodenal contents (including bile) to the lower esophagus may occur more frequently in patients undergoing gastrectomy than in the intact stomach.
This study examined whether reflux of duodenal juice to the esophagus is involved in esophageal carcinogenesis. We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/aoe-20-47).
Methods
We used 8-week-old male Wistar rats (250–300 g). We are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study complies with the ethical regulation of the use of laboratory animals and all experiments follow the animal experiment guideline in the Kindai University. All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of Kindai University (KAME-22-02).
Surgical procedure
Esophagoduodenal anastomosis (EDA) (n=27) (Figure 1 ) (4 )
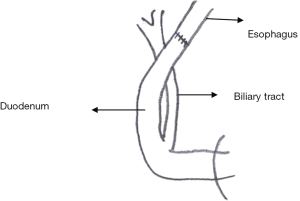
A total gastrectomy was performed and reconstruction was performed with an esophageal-duodenal anastomosis to create a model in which duodenal content refluxed into the esophagus. This procedure is the same as previously published (4).
Control rats (Control) (n=10); the sham operation, single laparotomy
Rats were sacrificed at 40th week after surgery under general anesthesia. The middle and lower esophageal tissues were collected, half were frozen at −80 °C and used for the measurement of oxidative stress, and the other half were fixed with 10% formalin and stained with HE and COX2. We measured bile acids in the common bile duct and in esophageal lavage fluid.
Biochemical assays of esophageal mucosa
Esophageal tissue frozen at −80 °C was used for tissue malondialdehyde (MDA) (µmol/L), glutathione (GSH) (mg/dL) and superoxide dismutase (SOD) (µmol/L). MDA was determined according to the method of Buege and Aust. GSH level was measured according to Saville’s method. SOD activity was determined by the method reported by Laihia.
Immunohistochemistry
Localisation of COX2 protein was determined by immunohistochemical staining using specific antibodies. The DAKO EnVision system (Dako Cytomation Japan CO. Ltd., Kyoto, Japan) was used with autoclave acceleration. Finally, the localization of COX2 was visualized with diaminobenzidine tetrahydrochloride.
Measurement of bile acid in the esophageal lumen and the common bile duct
The collected esophagus was rinsed with 0.5 cc of saline, the solution was centrifuged at 1,500 rpm at 4 °C for 5 min, and the supernatant was measured for bile.
Moreover, bile was collected by inserting a thin tube into the common bile duct.
We measured bile acid concentrations with the ENZa BILE kit (Daiichi Pure Chemicals, Tokyo).
Statistical analysis
Data are expressed as mean ± standard deviation (SD) of each group. Student’s t-test was used for statistical analysis. P<0.05 was considered statistically significant.
Results
Macroscopic findings
In the lower and middle esophagus in EDA rat, thickening and shortening of the wall were observed, and a small polypoid mass was present.
Microscopic finding (Figure 2)
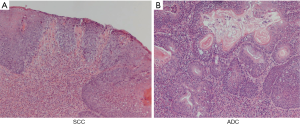
In EDA rat, 40 weeks after surgery, columnar-lined esophagus (CLE) was observed in 40%, SCC was observed in 40%, and adenocarcinoma (ADC) was observed in 30%.
Esophageal mucosal MDA (µmol/L), GSH (mg/dL) and SOD (µmol/L) activities (Figure 3)
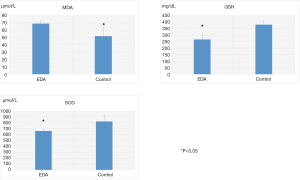
Mucosal MDA levels of EDA (69±4) were significantly higher compared to normal controls (52±11). GSH (267±50) and SOD levels (660±24) were significantly reduced in the EDA rat compared to the normal control rat (380±30, 822±104).
Immunohistochemistry of COX2 (Figure 4) (4)
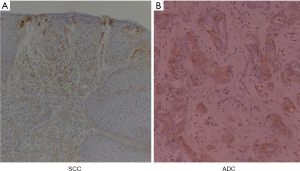
SCC and ADC were strongly stained with COX2 protein in EDA rat. COX2 staining was strong in dysplastic and cancerous esophageal mucosa obtained from EDA rat (4).
However, COX2 staining was not observed in the esophageal mucosa from the control rat.
Composition and measurement of bile collected from the common bile duct (Table 1)
Table 1
| Bile acid in common bile duct | EDA | Control | P value |
|---|---|---|---|
| Free bile acids | |||
| Cholic acid | 0.37±0.12 | 0.50±0.10 | – |
| Glycine conjugates | |||
| Glycocholic acid | 1.10±0.20 | 1.20±0.34 | – |
| Taurine conjugates | |||
| Taurocholic acid | 27.1±2.9 | 15.1±1.0 | <0.01 |
| Taurodeoxycholic acid | 2.28±0.43 | 1.20±0.30 | <0.01 |
| Taurochenodeoxycholic acid | 1.20±0.30 | 0.64±0.11 | <0.05 |
| Tauroursodeoxycholic acid | 0.45±0.10 | 0.20±0.03 | <0.01 |
| Total bile acid | 32.15±3.20 | 18.32±1.00 | <0.01 |
EDA, esophagoduodenal anastomosis.
Total bile acid in the esophageal lavage: EDA rat (175±50 µmol/L) was significantly higher compared to the control rat (35±5 µmol/L).
Total bile acids and bile acid components collected from the common bile duct: total bile acids of common bile duct in EDA rat was significantly higher than that of control rat. Moreover, from the point of bile acid composition in common bile duct, taurocholic acid (TCA), taurodeoxycholic acid (TDCA), taurochenodeoxycholic acid (TCDCA) and tauroursodeoxycholic acid (TUDCA) in EDA rat were significantly higher than those in control rat.
Discussion
Most cases of esophageal cancer are occupied by SCC or ADC. Forty years ago, 75% of esophageal cancer in the United States was SCC and the remaining 25% was ADC. Among white men, ADC frequency has increased sharply since mid-1970. And ADC now accounts for over 80% of esophageal cancer cases (5). The rapid increase in the frequency of ADC in the western world is due to the increased frequency of GERD and obesity.
Gastroesophageal reflux disease (GERD) and Barrett’s esophagus are major risk factors for ADC, and the risk of developing ADC increases with the frequency and duration of reflux symptoms. We believe that frequent reflux of gastroduodenal fluid containing acids, pepsin, and bile induces Barrett’s esophagus.
Some clinical reviews have highlighted the significance of chronic duodenal gastroesophageal reflux in the development of Barrett’s esophagus (6). In addition, chronic esophageal reflux of duodenal contents in our EDA model caused CLE, SCC and ADC.
Bile acids are known to promote gastrointestinal cancer growth, but the underlying mechanism is unknown. Recently, there have been many reports on the significance of bile acids in carcinogenesis.
Bile acids stimulate cell signaling effects including c-myc, COX2 and nuclear factor (NF)-κβ, and therefore, bile acids may be involved in carcinogenicity (7). Nehra et al. (8) collected and analyzed bile acids in the esophagus from patients without GERD and with GERD. They reported that TCA and TDCA were significantly higher concentrations in the reflux fluid of patients with erosive stenotic esophagitis. Chen et al. (9) reported that a high-fat diet increased the proportion of taurine conjugates, and chronic exposure of TCA to the esophagus caused tumor progression in a rat reflux model. Hong et al. (10) reported that TDCA significantly increased cell proliferation in EA cells. Piessen et al. (11) showed that TCA, TDCA, and TCDA were potent activators of MUC 4 expression. MUC4 (mucin 4) is a membrane-bound mucin that is overexpressed in the early stages of esophageal carcinogenesis and is involved in tumor progression. Zhang et al. (12) reported that TCA, TCDCA and TDCA individually, and also in a mixture, induced apoptosis of cultured human normal esophageal mucosal epithelial cells. These bile acids are involved in esophageal carcinogenesis. In our rat model, analysis of bile acids in the common bile duct of total gastrectomized rats also showed that TCA, TDCA and TCDCA and TUDCA were significantly higher than those in control rat. We suggest that TCA, TDCA, TCDCA and TUDCA play an important role of esophageal carcinogenesis. In contrast, Nishioka et al. (13) reported that chenodeoxycholic acid (CDCA) stimulates proliferation of esophageal squamous cell carcinoma (ESCC) cells. These results suggest that the effect of bile acids on cell proliferation varies with bile acid type and cell line studies. Shirvani et al. (14) suggest that bile acids stimulate COX2 expression. These phenomena are associated with chronic inflammation and proliferation in cancer and Barrett’s esophagus.
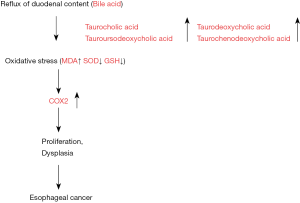
We confirmed that COX2 was strongly stained in cancer and atypical mucosa obtained from rats with esophageal duodenal anastomosis. In contrast, COX2 was not stained in esophageal mucosa from the control rat. Moreover, oxidative damage has been proposed as a possible mechanism for human GERD and possibly also CLE. Barrett’s esophagus is a complication of GERD. This specialized intestinal metaplasia is considered a premalignant condition of the esophageal carcinoma that is rapidly increasing in incidence. For the development of carcinoma, oxidative stress has been suggested to be a driving force. Furthermore, ROS can activate a number of cancer-associated signaling pathways such as PI3K/Akt, ERK1/2, and NF-κB (15). In EDA rats, MDA in the esophageal tissue was higher than that in control rat, and SOD and GSH in the esophageal tissue were much lower than those in control rat. In our study, lipid peroxidation in the esophageal epithelium was significantly higher in the EDA rat than that of control rat. These results indicate a possible mechanism by which bile acids (TCA, TDCA, TCDCA, TUDCA) dramatically increase intracellular ROS levels and subsequently induce COX2 in a rat model of duodenal esophageal reflux (Figure 5). The elucidation of the detailed mechanism by which bile acids induce COX2 may facilitate the development of chemoprevention strategies to reduce the risk of carcinogenesis in gastroesophageal tracts exposed to bile acids.
Conclusions
Bile reflux (TCA, TDCA, TCDCA, TUDCA) into the esophagus causes oxidative stress, subsequently induces COX2, and induces esophageal carcinogenesis.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The author has completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/aoe-20-47
Data Sharing Statement: Available at http://dx.doi.org/10.21037/aoe-20-47
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoe-20-47). NH serves as an unpaid editorial board member of Annals of Esophagus from Apr. 2019 to Mar. 2021. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study complies with the ethical regulation of the use of laboratory animals and all experiments follow the animal experiment guideline in the Kindai University. All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of Kindai University (KAME-22-02).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li HH, Zhang QZ, Xu L, et al. Clinical outcome of esophageal cancer after distal gastrectomy: a prospective study. Int J Surg 2008;6:129-35. [Crossref] [PubMed]
- Hirao M, Takiguchi S, Imamura H, et al. Comparison of Billroth I and Roux-en-Y reconstruction after distal gastrectomy for gastric cancer: one-year postoperative effects assessed by a multi-institutional RCT. Ann Surg Oncol 2013;20:1591-7. [Crossref] [PubMed]
- Hashimoto N, Inayama M, Fujishima M, et al. Esophageal cancer after distal gastrectomy. Dis Esophagus 2006;19:346-9. [Crossref] [PubMed]
- Hashimoto N. Effects of bile acids on cyclooxygenase-2 expression in a rat model of duodenoesophageal anastomosis. World J Gastroenterol 2014;20:6541-6. [Crossref] [PubMed]
- Abbas G, Krasna M. Overview of esophageal cancer. Ann Cardiothorac Surg 2017;6:131-6. [Crossref] [PubMed]
- Miwa K, Sahara H, Segawa M, et al. Reflux of duodenal or gastro-duodenal contents induces esophageal carcinoma in rats. Int J Cancer 1996;67:269-74. [Crossref] [PubMed]
- Song S, Guha S, Liu K, et al. COX-2 induction by unconjugated bile acids involves reactive oxygen species-mediated signalling pathways in Barrett's oesophagus and oesophageal adenocarcinoma. Gut 2007;56:1512-21. [Crossref] [PubMed]
- Nehra D, Howell P, Williams CP, et al. Toxic bile acids in gastro-oesophageal reflux disease: influence of gastric acidity. Gut 1999;44:598-602. [Crossref] [PubMed]
- Chen KH, Mukaisho K, Sugihara H, et al. High animal-fat intake changes the bile-acid composition of bile juice and enhances the development of Barrett's esophagus and esophageal adenocarcinoma in a rat duodenal-contents reflux model. Cancer Sci 2007;98:1683-8. [Crossref] [PubMed]
- Hong J, Behar J, Wands J, et al. Bile acid reflux contributes to development of esophageal adenocarcinoma via activation of phosphatidylinositol-specific phospholipase Cgamma2 and NADPH oxidase NOX5-S. Cancer Res 2010;70:1247-55. [Crossref] [PubMed]
- Piessen G, Jonckheere N, Vincent A, et al. Regulation of the human mucin MUC4 by taurodeoxycholic and taurochenodeoxycholic bile acids in oesophageal cancer cells is mediated by hepatocyte nuclear factor 1alpha. Biochem J 2007;402:81-91. [Crossref] [PubMed]
- Zhang R, Gong J, Wang H, et al. Bile salts inhibit growth and induce apoptosis of culture human normal esophageal mucosal epithelial cells. World J Gastroenterol 2005;11:6466-71. [Crossref] [PubMed]
- Nishioka K, Doki Y, Miyata H, et al. Bile acid promotes the proliferation of squamous cell carcinoma of the esophagus, independent of its inducing COX-2 expression. J Surg Res 2006;132:130-5. [Crossref] [PubMed]
- Shirvani VN, Ouatu-Lascar R, Kaur BS, et al. Cyclooxygenase 2 expression in Barrett's esophagus and adenocarcinoma: Ex vivo induction by bile salts and acid exposure. Gastroenterology 2000;118:487-96. [Crossref] [PubMed]
- Zhang F, Altorki NK, Wu YC, et al. Duodenal reflux induces cyclooxygenase-2 in the esophageal mucosa of rats: evidence for involvement of bile acids. Gastroenterology 2001;121:1391-9. [Crossref] [PubMed]
Cite this article as: Hashimoto N. Bile acids (taurocholic acid, taurodeoxycholic acid, taurochenodeoxycholic acid, tauroursodeoxycholic acid) develop esophageal cancer in a rat model of duodenoesophageal anastomosis after total gastrectomy. Ann Esophagus 2020;3:21.


