
Obesity and achalasia
Introduction
It seems counterintuitive to link obesity with a disorder that is classically associated with weight loss. However, there are a number of reasons this is an important discussion. Most importantly, there appears to be a significant, and independent, association between esophageal dysmotility and obesity, with higher BMI patients (BMI >50) exhibiting demonstrable, manometric dysmotility of the esophagus in 54% of patients in one study (1). Current data suggests that the prevalence of achalasia in a morbidly obese population is around 0.5–1% (2). Since we are dealing with an almost epidemic growth in obesity, then we are going to have to manage many more obese patients with achalasia (3). In a recent study, 70% of patients presenting with achalasia were classified as overweight or obese, a proportion very similar to that of a general western population (4).
The management of these patients is complex—we need to relieve dysphagia and yet promote healthy weight loss and resolution of co-morbid pathologies. Anecdotally, a number of the authors in this special issue have noted that over the course of their careers, patients presenting with achalasia appear to be getting ‘bigger’. There is concern that obesity may delay or hide the diagnosis, thereby leading to associated delays in management since it may be perceived that there is a lower severity given the preservation of total weight. Perhaps the increased availability of energy dense foods offsets the severity of calorie deficit at the time of presentation but these patients remain at risk of significant malnutrition. In one study, the Malnutrition Universal Screening Tool (MUST) was applied to achalasia patients, 50% of whom scored at moderate or high risk for malnutrition, equally spread between non-obese and obese patients (4). For a disorder so closely linked to food intake, there is a remarkable lack of research and data on the nutritional aspects of both presentation and management. Regardless, the longer the duration of symptoms, the more likely that there is an associated nutritional deficit.
Simultaneous treatment of achalasia and obesity
The best management for patients with temporally co-existing obesity and achalasia is an approach that combines the best treatment of both simultaneously. The problem with treating the achalasia component first is that this may result in further weight gain through relief of dysphagia. By far the most effective treatment of morbid obesity is bariatric surgery so that, although both pathologies can be managed by endoscopic methods, a combined surgical approach would be most practical, effective, and durable. This would also utilise procedures that lend themselves to combination—the set-up and positioning of laparoscopic abdominal ports needing little adjustment to facilitate either procedure. Perhaps the most natural combination therefore, is that of a laparoscopic Heller myotomy (LHM) with a standard laparoscopic Roux-en-Y gastric bypass (LRYGB). Kaufman et al. were the first to describe this simultaneous approach in 2005 (5). Since then a number of authors have replicated that technique. LRYGB in combination with an endoscopic approach to achalasia is also reasonable but again makes less sense when surgically both operations are on the same site.
Wesp and Farrell reviewed the present literature and noted that most experts support this simultaneous combination (6). Essentially, the myotomy provides relief of dysphagia and regurgitation through disruption of the lower esophageal sphincter and the bypass provides a low-pressure drainage system, with alleviation of reflux through bypassed duodeno-gastric juices. It results in a gastric pouch devoid of acid secreting parietal cells and a long Roux limb which prevents bile reflux. In addition, this results in powerful, effective and sustained weight loss through malabsorption. Since the stomach is not resected, should an esophagectomy be required for end-stage achalasia or even an associated esophageal cancer, then the gastric remnant could be used as a conduit (Figure 1).
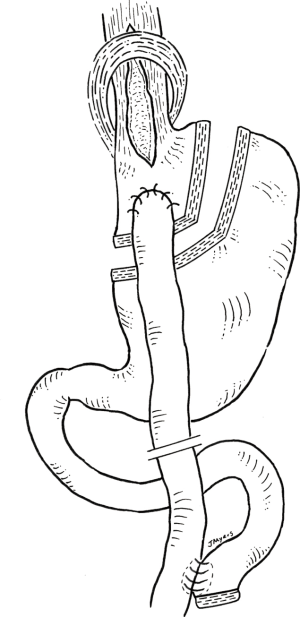
In contrast, though these may be technically easier to perform, the combination of LHM with the other standard bariatric procedures is less logical. For example, a laparoscopic sleeve gastrectomy and LHM would combine two procedures that are refluxogenic. In addition, sleeve gastrectomy can be associated with significant gastric dysphagia through restriction and a resulting high-pressure system. This would not lead to good results when trying to relieve dysphagia. Furthermore, the remnant stomach is discarded so the formation of a gastric conduit would not be possible should this be required. For similar reasons, laparoscopic gastric banding can result in significant dysphagia and would not be recommended in a patient with achalasia.
The less common bariatric surgical approaches, such as a duodenal switch or a biliary pancreatic diversion are not recommended. These are highly complex procedures with significant metabolic consequences that are only suitable for a select few patients. There are some newer procedures that may hold merit for combination in the future such as the single anastomosis loop duodenal switch (SADI-s/SIPS) but these remain experimental at present (7).
From a technical perspective, the LHM should be tackled first so that should a mucosal perforation occur then this can be easily dealt with by covering the myotomy with an anterior wrap and aborting the weight loss procedure. Equally, the author would avoid the passage of a bougie or an anvil following the myotomy due to the fragile nature of the single mucosal layer. An endoscope can be used to size the pouch and a handsewn or semi-mechanical anastomosis is the preferred gastro-jejunostomy technique.
Sequential treatment of achalasia and obesity
It is possible that achalasia can occur after a patient has had a bariatric surgical procedure. Equally, a patient who has been treated for achalasia can subsequently gain enough weight to render them morbidly obese. Finally, it is possible a patient may decline concurrent treatment of both achalasia and morbid obesity. In these situations, the treatments are therefore separated and sequential. Other factors now play a role in decision making.
Achalasia first
In this scenario, a patient has presented with achalasia and has had this treated. Bariatric surgery after prior endoscopic achalasia treatments such as POEM or balloon dilatation is easily achievable. In 2016, Oviedo et al. described a case of a young lady with achalasia who wanted to avoid a gastric bypass over concerns regarding malabsorption of vitamins and nutrients in the event of a future pregnancy. She ‘successfully’ underwent per-oral endoscopic myotomy (POEM) in preparation for a future laparoscopic sleeve gastrectomy, but the author believes the combination of endoscopic destruction of the LES mechanism and a sleeve gastrectomy is best avoided in view of the very high likelihood of postoperative reflux (over 50% risk of lifelong dependence on anti-reflux medication) (8). Although patients with obesity are no more likely to suffer reflux after POEM than non-obese patients, both groups of patients have a documented high incidence of reflux post-procedure (9).
However, in the scenario where a surgical LHM has been performed, further laparoscopic abdominal surgery can be challenging. All bariatric procedures involve access to the hiatus and the proximal stomach, essentially the same space as a previous myotomy and fundoplication. First, the subhepatic adhesions are divided. Second, the fundoplication is taken down to unfold the stomach prior to formation of a gastric pouch. Most surgeons prefer an anterior Dor fundoplication following a laparoscopic cardiomyotomy which lies across the front of the myotomy. Some surgeons prefer a posterior partial Toupet fundoplication. Regardless, the myotomy will be exposed during this dissection with a reasonable risk of a full-thickness perforation. The author recommends early insertion of an endoscope to confirm the location of the prior myotomy.
When creating the gastric pouch, care must be taken when firing the gastric stapler across the fundus. There is a risk of a leak from the staple line if the staples fail to be of the correct depth to cross any scar tissue. This risk would be substantially higher if the short gastric vessels had been previously taken. There is no simple solution so caution must be exercised in the dissection. For the reasons already discussed, a LRYGB would be the preferred bariatric surgical option, avoiding a high pressure, refluxo-genic sleeve. Endoscopic bariatric options such as placement of an intra-gastric balloon are absolutely contra-indicated and an endoscopic gastroplasty would suffer the same issues as a surgical sleeve (i.e., refluxogenic). There are also questions over its durability with the majority of studies reporting only 6 months of follow-up (10).
Obesity surgery first
Similar issues exist when performing a cardiomyotomy after bariatric surgery. Dealing with dense subhepatic adhesions and subsequent dissection of the hiatus can be very difficult. However, LHM is possible and has been shown to be an effective and safe therapy in patients post LRYGB (11). Despite being separated from the pouch, the gastric remnant can be brought across as a Dor fundoplication to cover the myotomy, but whether this has any anti-reflux function has not been studied (11). LHM after sleeve gastrectomy would also be feasible but again, this approach would carry a high reflux risk and is best avoided.
Endoscopic therapies are more attractive in this scenario. Intra-sphincteric botulinum toxin injection is the safest option but of only temporary benefit (see publication on Botulinum toxin injection in this special issue). However, pneumatic balloon dilatation and POEM are both excellent approaches in this situation with trends to better long-term outcomes with POEM over balloon dilatation (12,13).
The authors’ preference (assuming local expertise available) is a POEM in a patient who has had prior bariatric surgery. POEM is a very effective treatment, and a previous Roux-en-Y gastric bypass would act as an excellent anti-reflux combination. As such, the issues of post-POEM reflux are unlikely to be an issue in these patients (11).
Aiolfi et al. recently reviewed 12 studies with a total of 28 patients comparing surgical myotomy in 17 patients (61%) and POEM in 11 patients (39%): 61.5% had previously undergone laparoscopic RYGB and 38.5% an open RYBG. The elapsed time from the RYGB to myotomy ranged from 14 months to 14 years. Dysphagia (64%) and regurgitation (60.7%) were the most commonly reported symptoms. Postoperative morbidity was 3.6% and overall recurrence rate requiring re-operation was 7% but there were no significant differences between laparoscopic myotomy and POEM (14).
Summary
In summary, patients can develop achalasia if they are obese, and they can develop obesity if they have achalasia. In either scenario, they can have underlying malnutrition. The combination of a laparoscopic surgical myotomy and a standard Roux-en-Y gastric bypass is the most logical approach for both conditions if they present simultaneously. In a patient who has had their achalasia treated first, and then presents with morbid obesity, the authors’ preference is a laparoscopic Roux-en-Y bypass. In the patient who has had prior bariatric surgery, and then presents with achalasia, the authors’ preference is an endoscopic approach to treat the achalasia. This would entail pneumatic balloon dilatation or a POEM procedure, depending on local expertise.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Sarah Thompson) for the series “Achalasia” published in Annals of Esophagus. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/aoe-2019-ach-12). The series “Achalasia” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hong D, Khajanchee YS, Pereira N, et al. Manometric abnormalities and gastroesophageal reflux disease in the morbidly obese. Obes Surg 2004;14:744-9. [Crossref] [PubMed]
- Almogy G, Anthone GJ, Crookes PF. Achalasia in the context of morbid obesity: a rare but important association. Obes Surg 2003;13:896-900. [Crossref] [PubMed]
- Seidell JC, Halberstadt J. The global burden of obesity and the challenges of prevention. Ann Nutr Metab 2015;66:7-12. [Crossref] [PubMed]
- Newberry C, Vajravelu RK, Pickett-Blakely O, et al. Achalasia Patients Are at Nutritional Risk Regardless of Presenting Weight Category. Dig Dis Sci 2018;63:1243-9. [Crossref] [PubMed]
- Kaufman JA, Pellegrini CA, Oelschlager BK. Laparoscopic Heller myotomy and Roux-en-Y gastric bypass: a novel operation for the obese patient with achalasia. J Laparoendosc Adv Surg Tech A 2005;15:391-5. [Crossref] [PubMed]
- Wesp JA, Farrell TM. The Treatment of Achalasia in Obese Patients. Am Surg 2018;84:501-505. [Crossref] [PubMed]
- Brown WA, Ooi G, Higa K, et al. Single Anastomosis Duodenal-Ileal Bypass with Sleeve Gastrectomy/One Anastomosis Duodenal Switch (SADI-S/OADS) IFSO Position Statement. Obes Surg 2018;28:1207-16. [Crossref] [PubMed]
- Oviedo RJ, Sofiak CW, Dixon BM. Achalasia: A case report on its effect during surgical decision making for laparoscopic sleeve gastrectomy in the young morbidly obese patient. Int J Surg Case Rep 2016;26:4-6. [Crossref] [PubMed]
- Sanaka MR, Parikh MP, Subramanium S, et al. Obesity Does Not Impact Outcomes or Rates of Gastroesophageal Reflux After Peroral Endoscopic Myotomy in Achalasia. J Clin Gastroenterol 2020;54:338-43. [Crossref] [PubMed]
- Sartoretto A, Sui Z, Hill C, et al. Endoscopic Sleeve Gastroplasty (ESG) Is a Reproducible and Effective Endoscopic Bariatric Therapy Suitable for Widespread Clinical Adoption: a Large, International Multicenter Study. Obes Surg 2018;28:1812-21. [Crossref] [PubMed]
- Casas MA, Schlottmann F, Herbella FAM, et al. Esophageal achalasia after Roux-en-Y gastric bypass for morbid obesity. Updates Surg 2019;71:631-5. [Crossref] [PubMed]
- Bashir U, El Abiad R, Gerke H, et al. Peroral Endoscopic Myotomy Is Feasible and Safe in a Gastric Bypass Population. Obes Surg 2019;29:3523-6. [Crossref] [PubMed]
- Awaiz A, Yunus RM, Khan S, et al. Systematic Review and Meta-Analysis of Perioperative Outcomes of Peroral Endoscopic Myotomy (POEM) and Laparoscopic Heller Myotomy (LHM) for Achalasia. Surg Laparosc Endosc Percutan Tech 2017;27:123-31. [Crossref] [PubMed]
- Aiolfi A, Tornese S, Bonitta G, et al. Management of Esophageal Achalasia after Roux-en-Y Gastric Bypass: Narrative Review of the Literature. Obes Surg 2019;29:1632-7. [Crossref] [PubMed]
Cite this article as: Shenfine J. Obesity and achalasia. Ann Esophagus 2020;3:28.


