
Surgical treatment of Siewert type II gastroesophageal junction cancer: esophagectomy, total gastrectomy or other options?
Introduction
The molecular and anatomical origin of gastroesophageal junction (GEJ) cancer is, and has for decades, been a matter of intense debate, contributing to a lack of consensus regarding the optimal treatment of these patients. Siewert and Stein recognized the need for anatomical classification of GEJ cancer, and in an attempt to facilitate research and treatment proposed a system that classifies tumors into three categories based on anatomical location of the tumor center (1). The Siewert classification (Figure 1) has achieved great international acceptance by clinicians and is used almost universally around the world, directly impacting tumor staging and therapeutic strategies. Siewert type I tumors are generally considered to be esophageal cancer and treated as such with respect to neoadjuvant treatment and surgical approach, whereas type III are usually managed as gastric cancer (2). However, the optimal surgical approach for what is sometimes referred to as “true” GEJ tumors, those classified as Siewert type II, is still a matter of debate.
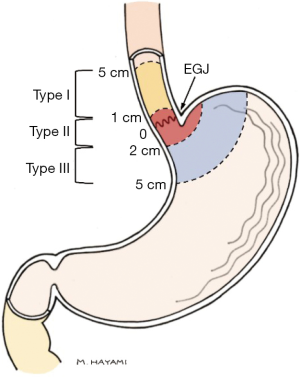
Difficulties in accurate classification of GEJ tumors complicates the choice between esophagectomy and gastrectomy. Endoscopy and endoscopic ultrasound (EUS) as well as computed tomography have a limited accuracy in correctly determining tumor location (3). Particularly large, bulky tumors can be difficult to classify, and even if correctly classified, the Siewert type can be irrelevant with regard to procedure choice (3,4). The primary long-term aim for any surgical procedure performed with curative intent for cancer is to remove the tumor completely, including regional lymph node disease, hence minimizing the risk for recurrent disease and optimizing overall survival (OS). Several studies comparing OS between gastrectomy and esophagectomy in GEJ cancer have not reported a survival difference amongst the two (5-7). Additionally, when comparing different approaches of gastrectomy and esophagectomy in GEJ cancer, no significant OS difference has been shown (8-11). The evidence suggests that one approach is not necessarily superior to the other in terms of OS. However, there might be differences in risk for postoperative complications, length of hospital stays (LOS), and disease-free survival as well as in number of harvested lymph nodes, that warrants further discussion. We therefore conducted a comprehensive review of the currently published literature, with the aim to guide in the decision between esophagectomy and gastrectomy for locally advanced, non-distant metastatic, GEJ cancer.
Surgical treatment of type II GEJ cancer
The main curatively intended treatment for GEJ cancer patients since many years is surgical resection of the tumor. Esophagectomy with resection of the proximal stomach or gastrectomy with distal esophagectomy are generally the options to choose from. Although esophagectomy is more commonly used in Western countries, while gastrectomy is often used in Japan, Korea and other Eastern Asian countries, the benefit of one over the other is unclear (11). In addition to this, there are different techniques for both procedures. Regardless of surgical approach, complete tumor removal with a negative resection margin (R0) has the highest prognostic significance and is the main objective in oncological surgery (12). Five-year survival for R0 resections range from 43–52% compared to 11–31% for positive resection margins (13,14). Furthermore, regional lymph nodes should be removed due to high risk of metastases, hence adequate lymphadenectomy is of high priority. The possible negative effect of extended lymphadenectomy may be increased surgical morbidity, making the balance between extensive and more limited lymph node dissection crucial. Selecting an appropriate surgical approach is important, not only to achieve a satisfactory oncological outcome, but also to minimize surgical trauma and its consequences for postoperative recovery and health-related quality of life.
Esophagectomy—do different approaches affect outcomes?
Esophagectomy is in Western countries by far the most common surgical procedure for type II GEJ cancer, however, due to challenges in Siewert classification in clinical practice, some type III cancer patients also undergo esophagectomies. The two principal esophagectomy approaches used are transthoracic esophagectomy, either Ivor Lewis or three stage McKeown types, and transhiatal esophagectomy (11).
A randomized clinical trial from the Netherlands compared transthoracic (n=114) with transhiatal esophagectomy (n=106) for GEJ cancer types I and II, in patients treated without neoadjuvant therapy. Perioperative morbidity was higher after transthoracic compared to transhiatal esophagectomy. Pulmonary complications occurred in 57% after transthoracic esophagectomy versus 27% after transhiatal procedure. Chyle leak as well as time in mechanical ventilator were significantly higher after transthoracic esophagectomies and in line with that, intensive care unit (ICU) stay and LOS were significantly longer in the transthoracic group. However, no difference was shown regarding in-hospital mortality between the surgical approaches. Mean lymph node yield was significantly higher with transthoracic compared to transhiatal esophagectomy (31±14 vs. 16±9 respectively, P<0.001). In patients with moderate numbers of node metastases (1–8 positive) loco-regional recurrence for patients operated with transthoracic technique had a 20% lower rate of local recurrence compared with transhiatal approach, however there was no difference in patients without, or those with more than 8, lymph node metastases (10). There was no difference in negative circumferential resection margin between the two groups. Neither DFS nor OS were significantly different between surgical approaches, however, later during follow-up the curves for both DFS and OS diverged showing a trend towards better survival following transthoracic esophagectomy, especially for Siewert type II cancers (9,10).
A prospective cohort study from the United Kingdom analyzed outcomes in 664 patients with type I or type II GEJ cancer undergoing transhiatal (n=263) or transthoracic (n=401) esophagectomy, including patients operated with right-sided thoracotomy and laparotomy (n=325) or left thoracoabdominal approach (n=76). Neoadjuvant chemotherapy was administered in 78.1% in the transhiatal group, and 47.1% in the transthoracic group. There was no difference in OS between surgical approaches, similarly there was no difference in time to tumor recurrence. Median lymph node yield was significantly higher with transthoracic approach (20 vs. 13, P<0.001). There was no difference regarding R0 resections, however, in a subgroup analysis of T3 and T4 tumors a trend towards a higher proportion of negative resection margins in the patients undergoing transthoracic esophagectomy was seen, although not statistically significant. In-hospital mortality was lower after transhiatal compared to the transthoracic esophagectomy, although it did not reach statistical significance, while median LOS was significantly shorter after transhiatal esophagectomy. No data was reported regarding postoperative complications (15).
A prospective cohort study using an American nationwide inpatient sample database analyzed 11,914 patients who underwent transhiatal esophageal resection and 5,481 patients undergoing transthoracic esophagectomy. There was no significant difference in LOS or overall morbidity, between transhiatal and transthoracic esophagectomy. Furthermore, there were no significant differences in the incidence of mediastinitis, wound infections, cardiovascular or pulmonary complications between the surgical approaches (16).
A cohort study identified 1,428 patients with esophageal cancer who were treated 2005-2011 using the American College of Surgeons‐National Surgical Quality Improvement Project (ACS‐NSQIP) database. Of them, 750 underwent transhiatal resection and 678 underwent transthoracic resection. Operative time was significantly longer in the transthoracic group and reoperation were more frequently needed, but LOS was similar in both groups. The frequency of overall serious morbidity was similar after transthoracic and transhiatal resections. Furthermore, there were no statistically significant differences in pulmonary, renal, cardiac, thromboembolic or septic complications. Postoperative 30-day mortality was slightly higher after transthoracic compared to transhiatal esophagectomy, however not with statistical significance (17).
A meta-analysis compared the outcomes of 7,527 patients undergoing either transhiatal or transthoracic esophagectomies for cancer of the esophagus or GEJ and did not show a difference in 3- or 5-year OS between surgical approach. In-hospital mortality was significantly higher after transthoracic compared to transhiatal resections. Pulmonary complications, chyle leak and wound infection was significantly higher in the transthoracic group whereas anastomotic leak and vocal cord damage were more frequent after transhiatal esophagectomy. Both ICU and LOS was significantly longer after transthoracic resection (18). Similarly, in a more recent meta-analysis comparing transthoracic and transhiatal resections for GEJ cancer, no OS survival difference was observed between surgical techniques. However, the 30-day mortality and hospital stay were higher in the transthoracic group than in the transhiatal group. Number of resected lymph nodes did not differ by surgical approach. Pulmonary complications were significantly higher in patients undergoing transthoracic resections, but no difference was seen in cardiovascular complications, and unlike the previously referred meta-analysis, anastomotic leaks did not differ by type of esophagectomy (19).
Our interpretation is that the standard choice, if esophagectomy is to be performed for a GEJ type II cancer, should be a two-stage Ivor Lewis esophagectomy with two field lymphadenectomy, as the weak available evidence indicates some oncological advantages and a low increase in operative risk with this procedure for fit patients. Transhiatal esophagectomy may mainly be used in patients where transthoracic approach entails a considerable increased perioperative risk because of comorbidity, particularly severe chronic pulmonary disease. Three stage esophagectomy according to McKeown can be required in a GEJ type II cancer if there are lymph node metastases in the upper mediastinum.
Gastrectomy
Total gastrectomy with D2 lymphadenectomy is also a valid treatment option for GEJ type II cancers and is widely used, especially in Asia (20,21).Together with gastrectomy transhiatal lymph node dissection of the lower mediastinum is possible but does not permit systematic dissection above the inferior pulmonary veins.
Proximal gastrectomy is mainly used in Asia for T1b tumors. It has been shown to have acceptable oncological outcome, although at most only a D1+ lymphadenectomy is possible. In a large retrospective cohort study 2,217 patients were included, 1,584 (71.4%) of the patients underwent total gastrectomy, and 633 (28.6%) of patients were treated with proximal gastrectomy. Overall, patients operated with total gastrectomy had a slightly higher OS compared to proximal gastrectomy, while DFS showed no statistically significant difference (22,23).
In a single-center retrospective cohort study 423 patients with type II or III GEJ cancer were operated with either proximal or total gastrectomy. There was no difference in 5-year OS between the procedures, but significantly more lymph nodes were retrieved after total gastrectomy of whom 12% experienced lymph node metastases compared to proximal gastrectomy, where 3.4% had metastatic nodes (24). A number of studies report severe problems with postoperative reflux after proximal gastrectomy (24,25). This problem has successfully been addressed with the use of jejunal interpositions (26) most recently represented by the double-tract reconstruction, and esophagogastric valve techniques (27,28).
Gastrectomy can be used to treat GEJ type II cancer. Locally advanced (cT2-cT4, node positive), GEJ type II cancers should be treated with total, rather than proximal, gastrectomy. Proximal gastrectomy with jejunal interposition, or esophago-gastric valve technique, can be used for early cancer. The limitation of the gastrectomy technique is the reduced opportunity for intrathoracic lymphadenectomy beyond the lower mediastinum.
Left thoracoabdominal esophagogastrectomy
A randomized controlled trial from Japan randomly assigned patients with type II or type III GEJ cancers into abdominal extended total gastrectomy with transhiatal lymphadenectomy in the lower mediastinum or left thoracoabdominal esophagogastrectomy. The study was terminated after the first interim analysis since the left thoracoabdominal group did not demonstrate improved survival compared to the abdominal gastrectomy group. The 5-year OS differed 14.4% in favor of the abdominal gastrectomy group; the difference not statistically significant. No difference was observed regarding overall complications, however, respiratory complications were significantly more frequent after left thoracoabdominal approach (8).
A retrospective cohort study, also from Asia, which included patients with type II GEJ cancer operated with left thoracoabdominal approach or abdominal gastrectomy, showed a trend towards better survival after abdominal approach, although not statistically significant. Some patients in both groups underwent proximal gastrectomy, however no subgroup analysis of these patients was reported. Patients undergoing abdominal gastrectomy had a higher number of retrieved lymph nodes, shorter operative time as well as a shorter LOS. Negative resection margin rate was similar in both groups. Postoperative complications were nearly twice as high after left thoracoabdominal compared to abdominal gastrectomy, 28.4% and 14.3% respectively, although no significant difference was observed in terms of 30-day postoperative mortality (21). Another large multicenter retrospective cohort study demonstrated reduced postoperative mortality and equal long-term and disease free survival comparing left thoracoabdominal esophagogastrectomy and Ivor Lewis esophagectomy for esophageal and junctional tumors (29).
Left thoracoabdominal esophagogastrectomy provides extremely good access to the GEJ. The technique has been associated with increased postoperative morbidity and long-term symptoms compared to abdominal gastrectomy, especially in Asian series, but the oncological results seem to be similar both to esophagectomy and gastrectomy. Large bulky tumors can be appropriate to operate with left thoracoabdominal technique, especially in obese, Western patients. Another situation is when the stomach is not available for reconstruction, and a long Roux-en-Y esophago-jejunostomy is needed. A limitation of the left thoracoabdominal approach is that it is so far not feasible with minimally invasive technique.
Which is the optimal surgical approach for GEJ type II cancer?
Whether the ideal procedure for type II GEJ tumors is esophagectomy, gastrectomy or left thoracoabdominal esophagogastrectomy is still unclear. All three procedures have been shown to yield similar oncologic outcomes and the optimal surgical approach remains controversial and the literature does not provide conclusive evidence.
Martin and colleagues published a large retrospective cohort study including 4,996 patients with type II GEJ cancers. The study included 1,181 patients of which 214 (18.2%) underwent gastrectomy and 967 (81.8%) esophagectomy. There were no differences concerning 30-day mortality or postoperative morbidity. Likewise, respiratory complications such as pneumonia and reintubation did not significantly differ by surgical approach. Another cohort study included 2,714 (71.1%) patients undergoing esophageal resection and 1,102 (28.9%) patients treated with gastrectomy. Median OS was significantly higher for patients undergoing esophagectomy compared to gastrectomy (26 vs. 21 months, P=0.025). However, after multivariable analysis surgical approach was not an independent predictor of OS (5).
A recently published retrospective cohort study analyzed perioperative and long-term outcomes in patients with type II GEJ cancer operated with total gastrectomy or esophagectomy. Approximately half of the patients had locally advanced tumors (cT3/4 or cN+) and received neoadjuvant chemotherapy. Overall complications, anastomotic leak, pulmonary complications and cardiac complications were similar. Tumor free resection margins, in-hospital mortality and 30-day mortality did not differ between gastrectomy and esophagectomy, although LOS was significantly higher after esophagectomy. Both surgical approaches had a median of 24 harvested nodes and no significant difference in median number of metastatic nodes was observed. The 5-year OS were significantly lower after gastrectomy compared to esophagectomy (57.5% vs. 69.6%, P=0.02), also 5-year DFS significantly favored esophagectomy (79.1% vs. 44.8%, P=0.002). The benefit for esophagectomy was seen in patients with cT3-T4 or clinically nodal positive disease, i.e., patients with a significant risk for lymph node metastases (30).
The results of a small European retrospective study, including patients with type II GEJ tumors that were not treated with neoadjuvant therapy, showed that recurrence free survival was significantly shorter after esophagectomy compared to gastrectomy. Furthermore, both univariable and multivariable Cox regression model analyses revealed that surgical approach was the strongest independent predictor of recurrence free survival and was in favor of gastrectomy (31).
A Dutch single center retrospective cohort study reviewed patients with GEJ cancer, of which 176 had GEJ type II tumors and compared the outcomes between gastrectomy and esophagectomy. There were no significant differences in 30-day or overall mortality between surgical approaches. No differences were seen concerning overall morbidity, pneumonia, or anastomotic leak. Median LOS and length of ICU stay were similar in both groups. Positive resection margin was significantly higher after gastrectomy compared to esophagectomy (29% vs. 11%, P=0.025) although 5-year OS showed no statistically significant difference. Moreover, no difference was observed in DFS or recurrence rate when comparing gastrectomy and esophagectomy (32).
A prospective cohort study compared negative resection margins, lymph node yield and survival in patients with type II and III GEJ cancer. Esophagectomy was performed in 155 patients and total gastrectomy in 85 patients. There was no statistically significant difference in incidence of non-radical resection margin between groups. Mean number of harvested lymph nodes was similar and no difference was observed between patients undergoing gastrectomy or esophagectomy concerning the 5-year OS (6). A retrospective study comparing esophagectomy and extended gastrectomy, in patients with GEJ cancer type I-III, did not show a significant difference in negative resection margin rate, LOS or ICU stay between the two procedures. No difference in survival was observed when comparing esophagectomy and gastrectomy in type I, II and III GEJ cancer, furthermore in subgroup analysis for patients with type II cancer no survival advantage was observed for patients in either surgical group (2).
Discussion
High grade scientific evidence to guide the optimal approach for surgical treatment of type II GEJ cancer is scarce. Basic surgical principles to ensure tumor free resection margins, adequate 2-field lymphadenectomy, and high-quality reconstruction determines which approach to choose. A type II GEJ tumor has a high probability for intrathoracic lymph node metastases making the transthoracic approach most appealing to many high-volume surgeons. Left thoracoabdominal esophagogastrectomy has, especially in Asian studies been associated with higher postoperative morbidity than abdominal total gastrectomy, but there are also reports of excellent results using this method (33). On the other hand, a well performed abdominal extended gastrectomy with resection of the lower mediastinal lymph nodes seem to yield similar results in the available literature (21,34).
Beside the differences between the above mentioned three distinct surgical options with regard to surgical morbidity and postoperative recovery, there are also major differences regarding which lymph node stations that can be cleared with each approach. Lymph node metastases is one of the strongest predictors of prognosis, despite the clear prognostic significance there is no consensus regarding extent of lymph node dissection for GEJ type II cancer. A prospective international multicenter study by Peyre and colleagues included 2,303 patients with esophageal cancer treated without neoadjuvant therapy and undergoing R0 resection (35). Median number of retrieved lymph nodes was 17 and the authors showed that the number of resected lymph nodes is an independent predictor of survival. The optimal threshold was ≥23 nodes harvested. Regardless of disease stage, survival was significantly better when this threshold was achieved, although the greatest benefit was seen in stage 3 disease. Furthermore, in stage II and stage III disease the 5-year survival rate increased with every 10 additional nodes resected (35). However, concerning patients treated with neoadjuvant therapy studies have shown that a higher number of retrieved lymph nodes did not correspond with an improvement in 5-year OS (36,37). In addition, a propensity-score-matched multicenter cohort study included patients with GEJ cancer receiving either neoadjuvant chemotherapy or nCRT followed by surgery showed that lymph node harvest did not affect survival or recurrence in the nCRT group. On the other hand, in the neoadjuvant chemotherapy group, a significant improvement was seen in both disease-free survival and overall recurrence rate when more than 52 lymph nodes were harvested, although not statistically significant, the OS was also improved in these patients (38).
In conclusion the scientific evidence is not strong enough to support a standardized surgical approach for patients with GEJ type II cancer. All three techniques; esophagectomy; gastrectomy; and left thoracoabdominal esophagogastrectomy, have strengths and weaknesses. Until better evidence is available the optimal approach should be tailored to the individual patient, and all three main surgical options, esophagectomy, abdominal total gastrectomy and left thoracoabdominal extended gastrectomy should be available at centers treating GEJ type II cancer. A weakness of many previous studies is the non-randomized design, introducing a selection bias between the approaches that can influence the results. The ongoing Cardia trial randomizes patients with type II GEJ cancer to esophagectomy or gastrectomy and will add important knowledge to the field once completed.
Acknowledgments
To Professor Riccardo Rosati for the invitation to write this review.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Riccardo Rosati) for the series “Current Issues on GEJ Adenocarcinoma” published in Annals of Esophagus. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/aoe-2020-geja-02). The series “Current Issues on GEJ Adenocarcinoma” was commissioned by the editorial office without any funding or sponsorship. MN serves as an unpaid editorial board member of Annals of Esophagus from Feb. 2018 to Jan. 2022. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg 1998;85:1457-9. [Crossref] [PubMed]
- Johansson J, Djerf P, Öberg S, et al. Two Different Surgical Approaches in the Treatment of Adenocarcinoma at the Gastroesophageal Junction. World J Surg 2008;32:1013-20. [Crossref] [PubMed]
- Grotenhuis BA, Wijnhoven BPL, Poley JW, et al. Preoperative Assessment of Tumor Location and Station-Specific Lymph Node Status in Patients with Adenocarcinoma of the Gastroesophageal Junction. World J Surg 2013;37:147-55. [Crossref] [PubMed]
- Curtis NJ, Noble F, Bailey IS, et al. The relevance of the Siewert classification in the era of multimodal therapy for adenocarcinoma of the gastro-oesophageal junction. J Surg Oncol 2014;109:202-7. [Crossref] [PubMed]
- Martin JT, Mahan A, Zwischenberger JB, et al. Should Gastric Cardia Cancers Be Treated with Esophagectomy or Total Gastrectomy? A Comprehensive Analysis of 4,996 NSQIP/SEER Patients. J Am Coll Surg 2015;220:510-20. [Crossref] [PubMed]
- Kauppila JH, Wahlin K, Lagergren J. Gastrectomy compared to oesophagectomy for Siewert II and III gastro-oesophageal junctional cancer in relation to resection margins, lymphadenectomy and survival. Sci Rep 2017;7:17783. [Crossref] [PubMed]
- Haverkamp L, Ruurda JP, van Leeuwen MS, et al. Systematic review of the surgical strategies of adenocarcinomas of the gastroesophageal junction. Surg Oncol 2014;23:222-8. [Crossref] [PubMed]
- Sasako M, Sano T, Yamamoto S, et al. Left thoracoabdominal approach versus abdominal-transhiatal approach for gastric cancer of the cardia or subcardia: a randomised controlled trial. Lancet Oncol 2006;7:644-51. [Crossref] [PubMed]
- Hulscher JBF, van Sandick JW, de Boer AGEM, et al. Extended Transthoracic Resection Compared with Limited Transhiatal Resection for Adenocarcinoma of the Esophagus. N Engl J Med 2002;347:1662-9. [Crossref] [PubMed]
- Omloo JMT, Lagarde SM, Hulscher JBF, et al. Extended Transthoracic Resection Compared With Limited Transhiatal Resection for Adenocarcinoma of the Mid/Distal Esophagus: Five-Year Survival of a Randomized Clinical Trial. Ann Surg 2007;246:992-1000. [Crossref] [PubMed]
- Kauppila JH, Lagergren J. The surgical management of esophago-gastric junctional cancer. Surg Oncol 2016;25:394-400. [Crossref] [PubMed]
- Klevebro F, Ekman S, Nilsson M. Current trends in multimodality treatment of esophageal and gastroesophageal junction cancer – Review article. Surg Oncol 2017;26:290-5. [Crossref] [PubMed]
- Feith M, Stein HJ, Siewert JR. Adenocarcinoma of the Esophagogastric Junction: Surgical Therapy Based on 1602 Consecutive Resected Patients. Surg Oncol Clin N Am 2006;15:751-64. [Crossref] [PubMed]
- O’Farrell NJ, Donohoe CL, Muldoon C, et al. Lack of Independent Significance of a Close (<1 mm) Circumferential Resection Margin Involvement in Esophageal and Junctional Cancer. Ann Surg Oncol 2013;20:2727-33. [Crossref] [PubMed]
- Davies AR, Sandhu H, Pillai A, et al. Surgical resection strategy and the influence of radicality on outcomes in oesophageal cancer. Br J Surg 2014;101:511-7. [Crossref] [PubMed]
- Connors RC, Reuben BC, Neumayer LA, et al. Comparing Outcomes after Transthoracic and Transhiatal Esophagectomy: A 5-Year Prospective Cohort of 17,395 Patients. J Am Coll Surg 2007;205:735-40. [Crossref] [PubMed]
- Papenfuss WA, Kukar M, Attwood K, et al. Transhiatal versus transthoracic esophagectomy for esophageal cancer: A 2005–2011 NSQIP comparison of modern multicenter results. J Surg Oncol 2014;110:298-301. [Crossref] [PubMed]
- Hulscher JBF, Tijssen JGP, Obertop H, et al. Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta-analysis. Ann Thorac Surg 2001;72:306-13. [Crossref] [PubMed]
- Wei MT, Zhang YC, Deng XB, et al. Transthoracic vs transhiatal surgery for cancer of the esophagogastric junction: A meta-analysis. World J Gastroenterol 2014;20:10183-92. [Crossref] [PubMed]
- Kim KT, Jeong O, Jung MR, et al. Outcomes of Abdominal Total Gastrectomy for Type II and III Gastroesophageal Junction Tumors: Single Center’s Experience in Korea. J Gastric Cancer 2012;12:36-42. [Crossref] [PubMed]
- Yang ZF, Wu DQ, Wang JJ, et al. Surgical approach for Siewert type II adenocarcinoma of the esophagogastric junction: transthoracic or transabdominal? —a single-center retrospective study. Ann Transl Med 2018;6:5. [Crossref]
- Zhu K, Xu Y, Fu J, et al. Proximal Gastrectomy versus Total Gastrectomy for Siewert Type II Adenocarcinoma of the Esophagogastric Junction: A Comprehensive Analysis of Data from the SEER Registry. Dis Markers 2019;2019:9637972 [Crossref] [PubMed]
- Yoo CH, Sohn BH, Han WK, et al. Proximal Gastrectomy Reconstructed by Jejunal Pouch Interposition for Upper Third Gastric Cancer: Prospective Randomized Study. World J Surg 2005;29:1592-9. [Crossref] [PubMed]
- An JY, Youn HG, Choi MG, et al. The difficult choice between total and proximal gastrectomy in proximal early gastric cancer. Am J Surg 2008;196:587-91. [Crossref] [PubMed]
- Yoo CH, Sohn BH, Han WK, et al. Long-term Results of Proximal and Total Gastrectomy for Adenocarcinoma of the Upper Third of the Stomach. Cancer Res Treat 2004;36:50-5. [Crossref] [PubMed]
- Kameyama J, Ishida H, Yasaku Y, et al. Proximal gastrectomy reconstructed by interposition of a jejunal pouch. Surgical technique. Eur J Surg 1993;159:491-3. [PubMed]
- Matsushiro T, Hariu T, Nagashima H, et al. Valvuloplasty plus fundoplasty to prevent esophageal regurgitation in esophagogastrostomy after proximal gastrectomy. Am J Surg 1986;152:314-9. [Crossref] [PubMed]
- Ahn SH, Jung DH, Son SY, et al. Laparoscopic double-tract proximal gastrectomy for proximal early gastric cancer. Gastric Cancer 2014;17:562-70. [Crossref] [PubMed]
- Davies AR, Zylstra J, Baker CR, et al. A comparison of the left thoracoabdominal and Ivor–Lewis esophagectomy. Dis Esophagus 2018;31:dox129 [Crossref] [PubMed]
- Blank S, Schmidt T, Heger P, et al. Surgical strategies in true adenocarcinoma of the esophagogastric junction (AEG II): thoracoabdominal or abdominal approach? Gastric Cancer 2018;21:303-14. [Crossref] [PubMed]
- Reeh M, Mina S, Bockhorn M, et al. Staging and outcome depending on surgical treatment in adenocarcinomas of the oesophagogastric junction. Br J Surg 2012;99:1406-14. [Crossref] [PubMed]
- Parry K, Haverkamp L, Bruijnen RCG, et al. Surgical Treatment of Adenocarcinomas of the Gastro-esophageal Junction. Ann Surg Oncol 2015;22:597-603. [Crossref] [PubMed]
- Markar SR, Schmidt H, Kunz S, et al. Evolution of Standardized Clinical Pathways: Refining Multidisciplinary Care and Process to Improve Outcomes of the Surgical Treatment of Esophageal Cancer. J Gastrointest Surg 2014;18:1238-46. [Crossref] [PubMed]
- Han WH, Eom BW, Yoon HM, et al. The optimal extent of lymph node dissection in gastroesophageal junctional cancer: retrospective case control study. BMC Cancer 2019;19:719. [Crossref] [PubMed]
- Peyre CG, Hagen JA, DeMeester SR, et al. The Number of Lymph Nodes Removed Predicts Survival in Esophageal Cancer: An International Study on the Impact of Extent of Surgical Resection. Trans Meet Am Surg Assoc 2008;126:190-7.
- Schaaf M van der, Johar A, Wijnhoven B, et al. Extent of Lymph Node Removal During Esophageal Cancer Surgery and Survival. JNCI J Natl Cancer Inst [Internet]. 2015 May [cited 2020 Mar 21];107(5). Available online: https://academic.oup.com/jnci/article-lookup/doi/10.1093/jnci/djv043
- Koen Talsma A, Shapiro J, Looman CWN, et al. Lymph Node Retrieval During Esophagectomy With and Without Neoadjuvant Chemoradiotherapy: Prognostic and Therapeutic Impact on Survival. Ann Surg 2014;260:786-92. [Crossref] [PubMed]
- Markar SR, Noordman BJ, Mackenzie H, et al. Multimodality treatment for esophageal adenocarcinoma: multi-center propensity-score matched study. Ann Oncol 2017;28:519-27. [Crossref] [PubMed]
Cite this article as: Saliba G, Hayami M, Klevebro F, Nilsson M. Surgical treatment of Siewert type II gastroesophageal junction cancer: esophagectomy, total gastrectomy or other options? Ann Esophagus 2020;3:18.


