Early distribution, clinical benefits, and limits of the implementation of the standardized clinical pathway following esophagectomy
Introduction
Esophageal cancer has been demonstrated to be the sixth most frequent malignancy leading to death worldwide. Esophageal cancer has been increasing in incidence until recently. Data from Asia has shown that incidence has peaked at over 400,000 new cases per year with similar plateauing incidence recently documented in Western countries (1). Incidence and mortality rates in the US have documented stable incidence figures for years but confirmed a slight decrease over the last decade, possibly related to Barrett’s surveillance programs. Evolution in incidence rates has also been associated with improvement in overall 5-year survival rate, with all cancer survival improving from approximately 5% of the 1960s–70s up to 20% currently (2).
The explanation for these changes over time are multifactorial but likely included advances in screening programs as well as endoscopic and surgical therapies. Treatment for regional esophageal cancer now centers on multimodal treatment, a more comprehensive and systematic approach, which also contributed to improving survival outcomes in locally advanced esophageal cancer (T2–3, N0–3, M0).
Esophagectomy has historically been widely accepted as the cornerstone of potentially curative treatment although postoperative outcomes have been associated with high morbidity and mortality rates. However, the introduction of new therapeutic, as well as process improvements, have contributed to decrease the morbidity after esophagectomy. Biere et al. conducted a multicenter, randomized controlled trial (3) assessing the incidence of pulmonary complications associated with the application of minimally invasive approaches, by surgeons experienced in both open and laparoscopic approaches and within high-volume hospitals (>30 esophagectomies/year). Similarly, overall mortality after esophagectomy has progressively decreased over the last decades, from 10–15% to recent reports demonstrating 90-day mortality as low as 4.5% (4,5). The incidence of perioperative complications has previously been poorly quantified due to the lack of a standardized reporting system. This was rectified recently with the publication of the Esophageal Complications Consensus Group (ECCG) documenting a contemporary international incidence for complications as 59% (5).
The complexity of this major surgical procedure, which involves both thoracic and abdominal components and requires a complex surgical reconstruction, has been associated with immediate and long-term side effects. In addition, patients often present as malnourished and with pre-existing comorbidities related to their advanced age. This complexity has been addressed in many countries by initiating a process of centralized high-risk cancer care. Many studies have previously demonstrated improved outcomes associated with treatment in high-volume centers (4) although the US has not yet initiated a program to centralize complex cancer care (6).
The measurement and audit of surgical perioperative outcomes require the general acceptance of a standardized system for reporting outcomes and quality measures which were accomplished in 2015 by the ECCG Report (7). Over time, outcomes have also been favorably impacted by the development and adoption of standardized clinical pathways (SCPs) and enhanced recovery after surgery (ERAS) guidelines to standardize the patients care pathway and goals. These pathways have also taken into account evolutions in surgical technique and multimodal care. SCPs have been previously demonstrated to significantly decrease perioperative mortality to <1% and improve the length of stay (LOS) to approximately 7–9 days (8,9). These SCPs have also been shown to be transferable to other high-volume centers (10). Overall standardized perioperative pathway aims to reduce the surgical impact and optimize recovery (11).
The best international example of the beneficial application of SCP has been developed by the ERAS Society which produced a clear and standardized approach to perioperative modules such as the prehabilitation and preoperative workup, intraoperative/immediate postoperative management and target infrastructure to avoid complications and promote discharge efficiency. The ERAS guidelines have been widely accepted in colorectal surgery and extended to other surgical fields such as gastrectomy, bariatric surgery, liver surgery, and gynecologic oncology. The ERAS guidelines for esophagectomy were published in February 2019 (12) utilizing many of the concepts validated in previous ERAS guidelines but expanding recommendations to cover areas unique to esophageal resection.
This article aims to review the current relevant literature in order to assess the impact of SCPs and ERAS on outcomes associated with esophagectomy and to assess the current levels of adoption in high volume programs.
Methods
A literature review was conducted through PubMed, Embase, Medline and Cochrane database search engines to identify relevant studies. Only comparative studies, randomized control trials (RCTs), as well as both prospective and retrospective cohort analyses, were identified on the basis of the following search mesh terms and keywords: “Esophagectomy” [Mesh], “Esophageal Neoplasms” [Mesh], “Critical Pathways” [Mesh], “Evidence-Based Medicine” [Mesh], “Evidence-Based Practice” [Mesh], Enhanced Recovery (or “Enhanced Recovery”), Fast Track (or Fast-Track or “Fast Track” or “Fast-Track”). Additional inclusion criteria included English-written articles analyzing the outcomes of an SCP in esophageal cancer surgery, regardless of whether ERAS or non-ERAS protocols were used. Studies concerning non-esophageal surgery or lacking any perioperative programs were excluded. All eligible articles were analyzed and categorized according to their primary postoperative outcomes: overall morbidity and postoperative complications, LOS and early mortality. All the selected articles were also reviewed to extract further variables of interest such as nutritional outcomes, in-hospital cost and SCP description, which could have potentially been included in the statistical analysis.
Results
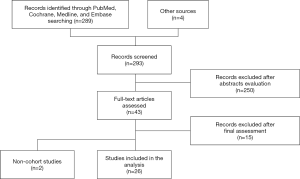
Literature search found 289 articles (Figure 1). Initial abstract evaluation and exclusion of non-English language publications ruled out 250 studies. After a comprehensive full-text assessment, the search resulted in 26 eligible articles (8,10,13-36) reporting on 3,721 patients. All reports included patients undergoing esophagectomy utilizing a variety of surgical approaches including transhiatal, left thoracoabdominal, 2- or 3-field procedures and minimally invasive or open technique. All 26 articles were comparative studies (five RCTs and six prospective trials) and reported outcomes associated with the application of SCP or ERAS programs. Information regarding the primary outcomes varied widely among selected studies while the insufficient collection of nutritional outcomes and in-hospital cost data did not enable a thorough analysis.
ERAS program and review articles protocols
Formalized ERAS recommendations have been recently published gathering all relevant perioperative items and introducing for the first time some components strictly applicable for esophagectomy. The new modules include procedure-specific components (such as preoperative nutritional assessment and treatment, preoperative oral pharmaco-nutrition and the adoption of both multidisciplinary tumor board and prehabilitation programs). Operative components (timing of surgery after neoadjuvant therapy, choice of conduit, pyloroplasty and extent of lymphadenectomy, use of peri-anastomotic or chest drain, NG tube and enteral feeding catheter, anesthetic management of fluid therapy and ventilation). However, the majority of the selected articles reviewed outcomes of their institutional version of SCP, which led to a variety of elements within the institutional protocols and variability in outcome assessment.
Postoperative outcomes
LOS
Twenty-four studies, for a total of 3,626 patients, reported data concerning the length of hospital stay. The mean LOS was lower in the SCP group (9.9±2.8) than in the control group (13.4±1.0) with a considerable difference which was also statistically significant (P<0.001). The readmission rate was also analyzed and found to be similar between groups (P=0.739).
Postoperative morbidity
Overall complications were reported in 19 studies comparing 1,129 and 962 patients within SCP and traditional group, respectively. The comprehensive incidences resulted in 29.8% and 32.5%, respectively, while the analysis also showed better rates in favor of the SCP group though the comparison did not reach statistical significance (P=0.350).
Postoperative mortality
In-hospital and 90-day mortality were randomly reported and their combined outcome was recorded among 17 studies involving 2,661 patients. The resulting mortality rate was 2.2% in the SCP group and 2.9% in patients managed by traditional methods. This difference did not achieve statistical significance (P=0.982).
Anastomotic leak
The dataset was also examined for specific complications and 19 studies (3,010 patients in total) reported the rate of anastomotic leak between groups: 8.3% and 10.3% were incidences of SCP and traditional group, respectively, which did not achieve statistical significance (P=0.659).
Pulmonary complication
Pulmonary complications were also reported in 17 studies involving 2,509 patients. Pulmonary complications occurred in the SCP group in 17.0% and in the traditional group 22.4%. The analysis showed that the adoption of SCP correlated with a statistically significant lower rate of pulmonary complications (P=0.011).
Discussion
Esophagectomy has been a historical outlier within oncologic operations due to a higher incidence of morbidity and mortality. SCPs and, more recently, ERAS guidelines are widely accepted as an effective approach to improve outcomes associated with esophagectomy. This analysis demonstrated that the application of SCP after esophagectomy has the potential to reduce LOS, morbidity and possibly mortality following esophageal resection. Moreover, there are statistically significant correlations between SCP and the incidence of pulmonary complications, which has historically been a targeted outcome parameter in clinical trials (37). This data supports the concept that SCPs positively impact perioperative outcomes. Although these results report only preliminary impressions, this review demonstrates that the incidence of clinical reports in the surgical literature are increasing, with more centers initiating personalized versions of SCPs and ERAS guidelines.
Impediments to initiating these programs include inadequate resources, resistance to change and staff training. Previously, there has been no standardized program that has resulted in centers introducing SCP according to their perceptions and their available resources. The recent publication of ERAS guidelines for esophagectomy has provided high-volume centers with a structured approach standardizing the fundamentals for infrastructural changes for centers wishing to initiate or expand their ERAS programs.
Once SCPs or ERAS programs have been initiated, there needs to be a regular audit of “critical pathway goals” to ensure adherence and maintenance of ERAS guidelines. When non-adherence and deviation from critical goals are identified, it can be addressed. The greatest vulnerability for long-term maintenance of SCPs and ERAS programs is staff turnover in key areas of the multidisciplinary team. This must be monitored and ongoing orientation and education are regular components of successful programs.
The greatest impediment to the initiation of successful programs is the willingness of key personnel to modify their traditional approach to service delivery and adopt new techniques within SCP or ERAS programs. Previous articles have documented a “resistance to change” due to a disinclination to adopt these working practices.
This clinical review confirms the potential clinical benefits associated with the adoption of SCP and ERAS protocols to standardize perioperative management of patients undergoing esophagectomy, however, this assessment does have certain inherent limitations. The report involved the review of a variety of experiences from centers applying a variable approach to ERAS and SCPs. This inevitably leads to heterogeneity among selected studies and within themselves, which likely affected the analysis. For instance, most of the included trials focused on results following non-specific surgical procedures while only 5 of them (3 prospective and 2 retrospectives) strictly selected patients according to the type of surgery (3 open Ivor Lewis, 1 left-thoracoabdominal and 1 minimally invasive 3-field esophagectomy). Further variability was due to the application of different SCP components across all reports. These dissimilarities, which resulted from the examination of all protocols, highlight current differences between SCPs and ERAS programmatic goals.
Another possible bias was also the unclear definition of most of the postoperative complications which were often reported according to personal definitions rather in a standardized fashion as published by the ECCG (7). Nevertheless, this comprehensive review of the current literature was clearly able to confirm the important positive influence that SCPs and ERAS can have on the outcomes of esophagectomy.
In conclusion, the application of SCP or ERAS protocols in esophageal surgery has been demonstrated to produce measurable clinical advantages on postoperative outcomes, such as lower morbidity and significantly shorter LOS. However, there is still a need for more studies specifically focusing on the current ERAS guidelines following specific esophagectomy procedures, and assessing the long-term feasibility of maintaining these pathways in the context of high-volume centers.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Fernando A. M. Herbella, Rafael Laurino Neto and Rafael C. Katayama) for the series “How Can We Improve Outcomes for Esophageal Cancer?” published in Annals of Esophagus. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoe.2020.02.06). The series “How Can We Improve Outcomes for Esophageal Cancer?” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- International Agency for Research on Cancer. CI5: Cancer incidence in five continents. Available online: http://ci5.iarc.fr/Default.aspx
- NIH. Cancer stat facts: esophageal cancer. Available online: https://seer.cancer.gov/statfacts/html/esoph.html. Accessed on Jan 2019.
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Reames BN, Ghaferi AA, Birkmeyer JD, et al. Hospital volume and operative mortality in the modern era. Ann Surg 2014;260:244-51. [Crossref] [PubMed]
- Low DE, Kuppusamy MK, Alderson D, et al. Benchmarking complications associated with esophagectomy. Ann Surg 2019;269:291-8. [Crossref] [PubMed]
- Munasinghe A, Markar SR, Mamidanna R, et al. Is it time to centralize high-risk cancer care in the United States? Comparison of outcomes of esophagectomy between England and the United States. Ann Surg 2015;262:79-85. [Crossref] [PubMed]
- Low DE, Alderson D, Cecconello I, et al. International Consensus on standardization of data collection for complications associated with esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015;262:286-94. [Crossref] [PubMed]
- Markar SR, Schmidt H, Kunz S, et al. Evolution of standardized clinical pathways: refining multidisciplinary care and process to improve outcomes of the surgical treatment of esophageal cancer. J Gastrointest Surg 2014;18:1238-46. [Crossref] [PubMed]
- Low DE, Kunz S, Schembre D, et al. Esophagectomy--it's not just about mortality anymore: standardized perioperative clinical pathways improve outcomes in patients with esophageal cancer. J Gastrointest Surg 2007;11:1395-402; discussion 402. [Crossref] [PubMed]
- Preston SR, Markar SR, Baker CR, et al. Impact of a multidisciplinary standardized clinical pathway on perioperative outcomes in patients with oesophageal cancer. Br J Surg 2013;100:105-12. [Crossref] [PubMed]
- Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg 2017;152:292-98. [Crossref] [PubMed]
- Low DE, Allum W, De Manzoni G, et al. Guidelines for perioperative care in esophagectomy: enhanced recovery after surgery (ERAS((R))) Society Recommendations. World J Surg 2019;43:299-330. [Crossref] [PubMed]
- Zhang Z, Zong L, Xu B, et al. Observation of clinical efficacy of application of enhanced recovery after surgery in perioperative period on esophageal carcinoma patients. J BUON 2018;23:150-56. [Crossref] [PubMed]
- Taniguchi H, Sasaki T, Fujita H, et al. Effects of goal-directed fluid therapy on enhanced postoperative recovery: an interventional comparative observational study with a historical control group on oesophagectomy combined with ERAS program. Clin Nutr ESPEN 2018;23:184-93. [Crossref] [PubMed]
- Akiyama Y, Iwaya T, Endo F, et al. Effectiveness of intervention with a perioperative multidisciplinary support team for radical esophagectomy. Support Care Cancer 2017;25:3733-39. [Crossref] [PubMed]
- Giacopuzzi S, Weindelmayer J, Treppiedi E, et al. Enhanced recovery after surgery protocol in patients undergoing esophagectomy for cancer: a single center experience. Dis Esophagus 2017;30:1-6. [Crossref] [PubMed]
- Cooke DT, Calhoun RF, Kuderer V, et al. A defined esophagectomy perioperative clinical care process can improve outcomes and costs. Am Surg 2017;83:103-11. [PubMed]
- Li W, Zheng B, Zhang S, et al. Feasibility and outcomes of modified enhanced recovery after surgery for nursing management of aged patients undergoing esophagectomy. J Thorac Dis 2017;9:5212-19. [Crossref] [PubMed]
- Raman V, Kaiser LR, Erkmen CP. Clinical pathway for esophagectomy improves perioperative nutrition. Healthc (Amst) 2016;4:166-72. [Crossref] [PubMed]
- Chen L, Sun L, Lang Y, et al. Fast-track surgery improves postoperative clinical recovery and cellular and humoral immunity after esophagectomy for esophageal cancer. BMC Cancer 2016;16:449. [Crossref] [PubMed]
- Karran A, Wheat J, Chan D, et al. Propensity score analysis of an enhanced recovery programme in upper gastrointestinal cancer surgery. World J Surg 2016;40:1645-54. [Crossref] [PubMed]
- Gatenby PA, Shaw C, Hine C, et al. Retrospective cohort study of an enhanced recovery programme in oesophageal and gastric cancer surgery. Ann R Coll Surg Engl 2015;97:502-7. [Crossref] [PubMed]
- Wang JY, Hong X, Chen GH, et al. Clinical application of the fast track surgery model based on preoperative nutritional risk screening in patients with esophageal cancer. Asia Pac J Clin Nutr 2015;24:206-11. [PubMed]
- Bhandari R, Hao YY. Implementation and effectiveness of early chest tube removal during an enhanced recovery programme after oesophago-gastrectomy. JNMA J Nepal Med Assoc 2015;53:24-7. [Crossref] [PubMed]
- Shewale JB, Correa AM, Baker CM, et al. Impact of a fast-track esophagectomy protocol on esophageal cancer patient outcomes and hospital charges. Ann Surg 2015;261:1114-23. [Crossref] [PubMed]
- Pan H, Hu X, Yu Z, et al. Use of a fast-track surgery protocol on patients undergoing minimally invasive oesophagectomy: preliminary results. Interact Cardiovasc Thorac Surg 2014;19:441-7. [Crossref] [PubMed]
- Findlay JM, Tustian E, Millo J, et al. The effect of formalizing enhanced recovery after esophagectomy with a protocol. Dis Esophagus 2015;28:567-73. [Crossref] [PubMed]
- Ford SJ, Adams D, Dudnikov S, et al. The implementation and effectiveness of an enhanced recovery programme after oesophago-gastrectomy: a prospective cohort study. Int J Surg 2014;12:320-4. [Crossref] [PubMed]
- Zhao G, Cao S, Cui J. Fast-track surgery improves postoperative clinical recovery and reduces postoperative insulin resistance after esophagectomy for esophageal cancer. Support Care Cancer 2014;22:351-8. [Crossref] [PubMed]
- Tang J, Humes DJ, Gemmil E, et al. Reduction in length of stay for patients undergoing oesophageal and gastric resections with implementation of enhanced recovery packages. Ann R Coll Surg Engl 2013;95:323-8. [Crossref] [PubMed]
- Blom RL, van Heijl M, Bemelman WA, et al. Initial experiences of an enhanced recovery protocol in esophageal surgery. World J Surg 2013;37:2372-8. [Crossref] [PubMed]
- Li C, Ferri LE, Mulder DS, et al. An enhanced recovery pathway decreases duration of stay after esophagectomy. Surgery 2012;152:606-14; discussion 14-6. [Crossref] [PubMed]
- Cao S, Zhao G, Cui J, et al. Fast-track rehabilitation program and conventional care after esophagectomy: a retrospective controlled cohort study. Support Care Cancer 2013;21:707-14. [Crossref] [PubMed]
- Munitiz V, Martinez-de-Haro LF, Ortiz A, et al. Effectiveness of a written clinical pathway for enhanced recovery after transthoracic (Ivor Lewis) oesophagectomy. Br J Surg 2010;97:714-8. [Crossref] [PubMed]
- Tomaszek SC, Cassivi SD, Allen MS, et al. An alternative postoperative pathway reduces length of hospitalisation following oesophagectomy. Eur J Cardiothorac Surg 2010;37:807-13. [Crossref] [PubMed]
- Zehr KJ, Dawson PB, Yang SC, et al. Standardized clinical care pathways for major thoracic cases reduce hospital costs. Ann Thorac Surg 1998;66:914-9. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
Cite this article as: Puccetti F, Kuppusamy MK, Hubka M, Low DE. Early distribution, clinical benefits, and limits of the implementation of the standardized clinical pathway following esophagectomy. Ann Esophagus 2020;3:7.