
Esophagectomy for cancer in the obese patient
Obesity and cancer
Most of the world’s population lives in countries where overweight and obesity are prevalent causes of morbidity and mortality (1). Indeed, the worldwide prevalence of obesity has almost tripled in the past fifty years: between 1975 and 2014, the age-standardized prevalence has increased from 3.2% to 10.8% in men, and from 6.4% to 14.9% in women, respectively (2).
Obesity is a demonstrated risk factor for several chronic diseases (such as hypertension, type 2 diabetes, dyslipidemia, coronary artery disease) and in the past decade it was demonstrated that obesity not only predisposes for certain types of cancer (3) (Table 1), but also plays a role as a negative prognostic factor in certain oncological patients (17,18). Therefore, the International Agency for Research on Cancer (IARC) published in 2002 a monography focused on weight control and physical activity as cancer-preventive strategies (19).
Table 1
| Strength of evidence | Cancer type |
|---|---|
| Convincing | Esophageal adenocarcinoma ( |
| Pancreatic cancer ( |
|
| Liver cancer ( |
|
| Colorectal cancer ( |
|
| Postmenopausal breast cancer ( |
|
| Endometrial cancer ( |
|
| Renal cell carcinoma ( |
|
| Thyroid cancer ( |
|
| Multiple myelomas ( |
|
| Meningioma ( |
|
| Probable | Mouth, pharynx and larynx cancer ( |
| Gastric (cardia) cancer ( |
|
| Gallbladder cancer ( |
|
| Ovarian cancer ( |
|
| Advanced-stage prostate cancer ( |
“Convincing” and “probable” are used with the same meaning reported by the World Cancer Research Fund/American Institute for Cancer Research in its annual reports (Available online at
Obesity and esophageal cancer
Seventeen thousand six hundred and five cases of esophageal cancer are on average diagnosed every year in the United States, as well as 16,080 related deaths are reported (20). According to data from the GLOBOCAN database (21), an estimated 572,034 new cases and 508,585 deaths are expected on a worldwide scale annually.
Interestingly, the temporal trend in incidence has varied for the two major histologic types of esophageal cancer, which are adenocarcinoma and squamous cell carcinoma (SCC).
Incidence rates for adenocarcinoma have been increasing dramatically in the majority of the Western countries, mainly due to an increased prevalence of its known risk factors such as overweight and obesity (22).
Incidence rates for SCC have been steadily decreasing in these same countries because of long-term reductions in tobacco and alcohol consumption and also because obesity has been proven to have a paradoxical, inverse association with SCC development (23).
The mechanisms underlying the strong positive associations between obesity and esophageal adenocarcinoma have not been completely clarified yet. The two major hypothesis, equally valuable from a pathophysiologic standpoint, concern the mechanical effects of abdominal obesity in promoting gastroesophageal reflux disease (GERD) on one hand (24), and obesity-related metabolic pathways alterations on the other hand. GERD, a well-known risk factor for the development of Barrett’s esophagus (25,26), which is the precursor of esophageal adenocarcinoma (27), is more prevalent with higher BMI, due to mechanically increased intra-abdominal pressure (28-30).
Metabolic pathways that modulate cell proliferation, apoptosis and cell growth involve molecules that have been proven to be altered in obesity (31), in which insulin-resistance, pro-inflammatory cytokines and adipokine seem to play a central role (32-39). However, the way these pro-inflammatory processes interplay with Barrett’s esophagus in promoting esophageal metaplasia, dysplasia and eventually carcinogenesis still have to be elucidated.
To sum up, compared to normal weight, overweight is associated with a 1.5–2 folds risk of developing esophageal adenocarcinoma, while obesity has a 2–3 folds increased risk (40).
With regards to the inverse association between obesity and SCC, the underlying biological mechanisms are still unclear, despite extensive investigation (40). Moreover, the association itself is a source of debate in the literature since it was observed in smokers only and not in former or never smokers (41)—which led to the hypothesis that the inverse association may be simply the result of a residual confounding factor, since smoking is notably predisposing for the development of esophageal SCC (42,43).
Esophagectomy in the obese patients
The surgical management of esophageal cancer in patients with BMIs in the range of obesity presents specific challenges.
Surgical planning
Challenges with obese patients may begin far from the operating table, during the acquisition of pre-operative images. Indeed, obesity has a well-known impact on imaging gaining.
Patients who exceed the weight limit of the CT/MRI table as defined by the manufacturer or who exceed the gantry diameter because of their girth may end up not receiving a pre-operative imaging assessment, which contraindicates any further surgical approach.
Provided a patient fits the weight and girth to proceed with a CT scan, the quality of the obtained images may be impaired due to some technical limitations related to the exceeding amount of adipose tissue: increased noise due to inadequate beam penetration, limited field of view, image cropping are some of the reported issues to deal with (44). However, it has also been reported that patients who have predominantly intraperitoneal or retroperitoneal fat have an enhanced visualization of internal organ structures because of better delineation by the surrounding fat, if compared to patients with a scarceness of intraperitoneal adipose tissue (44) (Figure 1).
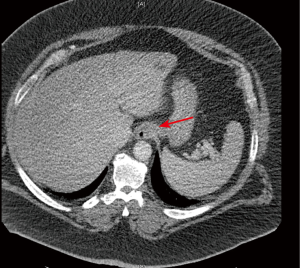
With regards to MRI, typically MR scanners with a high signal-to-noise ratio and strong gradients (1.5 T) have a limit of 350 lb (159 kg) in weight or 60 cm in diameter (45). Vertical field open MRIs allow for weight accommodation up to 550 lb (250 kg), but have a lower signal-to-noise ratio and weaker gradients (45). Analogously to CT scan, limitations of MRI in obese patients include altered radiofrequency penetration and gradient strength, limited field of view, augmented scanning time and radiofrequency energy deposition on the skin where it abuts the gantry (44). Manufacturers of CT and MRI machines make new design changes on a yearly basis in order to accommodate obese patients, so this limitation on imaging assessment may no longer be an open issue in the upcoming years.
Anesthesia considerations
The anesthetic management of elective esophageal surgery faces an increased risk for pulmonary aspiration, possible need for one lung ventilation (OLV) and requires a careful post-operative pain management.
Minimizing the risk of pulmonary aspiration is essential, especially taking into account that patients with esophageal cancer have a baseline increased risk due to the presence of the esophageal mass leading to stricture and motility abnormality. In addition to that, obese patients have a higher chance of gastro-esophageal reflux because of the augmented intra-abdominal pressure (46,47). Therefore, when planning to induce general anesthesia, it is important to educate the patient on the fasting requirement prior to surgery (48); precautions also include a rapid sequence induction and intubation (RSII) technique, which has proven to protect the airway and minimize the chance of aspiration (49,50).
The airway management of obese patients is considered challenging for several reasons: first, because restricted lung mechanisms due to excessive adiposity lead to a reduced functional residual capacity and to ventilation-perfusion mismatch (51-56); second, because of obesity-related respiratory comorbidities, namely airway hyper-reactivity (57-59), sleep apnea (60-62) and obesity hypoventilation syndrome (63-65); finally, because of a higher risk of post-operative respiratory depression related to the adiposity-altered pharmacokinetics.
Thus, it is extremely important to determine during the pre-anesthetic planning whether OLV is necessary, and which instrument should be used amongst the variety of existing double-lumen endotracheal tubes and bronchial blockers. Obesity poses additional challenges in achieving adequate protective ventilation through OLV, but OLV has been demonstrated to be adequate and effective (66). Several studies on OLV (although not performed in obese patients undergoing esophagectomy) recommended a lung protective strategy with a tidal volume of 4–6 mL/kg of predicted (not actual) body weight (67-69), and suggested alveolar recruitment strategies in order to decrease dead-space variables and enhance oxygenation (70). Practical recommendations for intraoperative ventilation of obese patients are summarized in Figure 2.
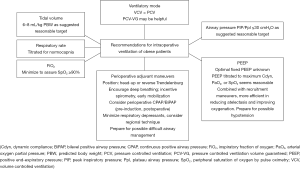
Post-operative pain control has to be carefully planned, too. Thoracic epidural analgesia, paravertebral bloc, erector spinae block, or other regional anesthetic options have to be taken into account to achieve optimal pain management. Vigilant dose adaptation according to the weight is necessary, since these patients may experience altered pharmacokinetics due to malabsorption, abnormal distribution, and altered clearance (71,72).
Intra-operative considerations and peri-operative care
The indications for esophagectomy as first therapeutic line or for esophagectomy following neoadjuvant chemo(radiation) therapy do not differ in obese patients, and can be consulted in the latest version of the NCCN Clinical Practice Guidelines for esophageal and esophagogastric junction (EGJ) cancers (73).
This review does not cover the management of obese patients with unresectable esophageal cancer.
Adiposity and obesity have proven to be associated with increased morbidity after general and esophageal surgery (74). However, studies analyzing long-term outcomes after esophagectomy have surprisingly demonstrated a better long-term survival in obese patients (75-77) and this was recently confirmed by a meta-analysis (78). Therefore, obesity should not be considered a contraindication for esophagectomy.
In the operating room, obese patients require specific attention in terms of surgical planning, equipment needed, and number of people involved in taking care of them before, during and after the surgical procedure. This is even more actual when esophagectomy is performed with minimally invasive techniques, as potentially longer operative time and more advanced technical skills may be required. Ports placement needs to be customized according to the body shape (apple-shaped vs. pear-shaped obesity) and suturing the trocar holes at the end of the procedure requires particular awareness in order to avoid the formation of subcutaneous seroma, decubitus or hernias. If truncal adiposity is predominant, laparoscopic instruments and/or robotic arms reaching the surgical target may be impinged by the impaired degree of mobility allowed by the thick adipose layers. Moreover, an augmented amount of intra-peritoneal fat may subvert the shape and placement of the organs, thus leading to a higher risk of injuring noble anatomical structures.
Despite all the aforementioned procedural challenges, obese patients undergoing esophagectomy can definitely benefit from a minimally invasive approach. Indeed, established evidence has related minimally invasive esophagectomy to lower pulmonary complication rates than an open approach, with no negative impact on the oncologic outcome (79,80).
The peri-operative care involves a careful assessment of the obese patient to prevent the development of morbid conditions related to surgery in general, and to esophagectomy in particular. Patients require systemic antibiotic prophylaxis and deep venous thrombosis prophylaxis to prevent infectious or thrombo-embolic complications, respectively.
Adequate enteral nutritional intake has to be maintained through a jejunostomy tube (JT), which is placed intraoperatively. The JT is used for enteral feeding starting on post-operative day 2, then advanced until feeding goals are achieved. Interestingly, Fenton et al. have reported a significantly higher odds of being discharged with the JT being used in underweight patients (BMI <18.5 kg/m2) compared with obese (BMI >30 kg/m2) patients (odds ratio, 7.56; 95% confidence interval, 1.19 to 48.03) (81). It is important to perform a barium swallow or a CT with oral contrast for any reason to suspect an anastomotic dehiscence, always keeping in mind that obese patients hold a higher risk for anastomotic leak and that prompt diagnosis is key for rescuing the patient (78). New onset of atrial fibrillation should always give rise to suspicion of anastomotic leak, especially in obese patients with diabetes where it has found to be more frequent than in obese patients without diabetes (82).
To summarize, surgeons should maintain a lower threshold in obese patients for the investigation of complications, ensuring a scrupulous management of comorbidities throughout the peri-operative period.
Is there a role for weight-loss surgery?
After esophagectomy, most surgeons use a gastric conduit to replace the esophagus and restore gastrointestinal continuity. Alternative conduits include the colon or jejunum as a Roux-en-Y reconstruction. This significantly overlaps, from a conceptual standpoint, the field of bariatric surgery. Interestingly, Ouattara et al. have investigated the kinetics of weight loss after esophagectomy (83). Tracking BMI changes over time, they reported that esophageal cancer surgery seemed to have a substantial bariatric effect (Figure 3).
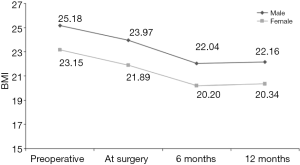
They also focused on assessing malnutrition before and after esophagectomy in terms of unintentional weight loss, which is one of the most manifest side effects 6 months postoperatively (84). Notably, preoperative overweight and obesity have proved to be independent factors for post-esophagectomy malnutrition (85,86). A special vigilance on the nutritional status of these patients is therefore indicated. According to our experience, an experienced Bariatric Surgeon should be involved in the multidisciplinary team approaching the obese patient with esophageal cancer—and the ultimate decision whether to perform a bariatric procedure prior to the esophagectomy has to be made taking into account the patient’s BMI, comorbidities, oncological and psychological status.
Indeed, esophagectomy can be safely performed (also in a minimally invasive fashion) in those patients who had received gastric bypass—as it is well tolerated and technically feasible, and leads to acceptable oncologic outcomes (87). Although there is no abundance of literature investigating the role of bariatric surgery prior to esophagectomy, Roux-en-Y gastric bypass would be the preferred choice for weight-reduction surgery in these patients, as the stomach could still be used later as a conduit for reconstructing the alimentary canal continuity (88). In this case, the Roux limb has to be divided a few centimeters below the gastric pouch. Dissection of the celiac nodes should be performed, and the left gastric vessels should be divided. The remaining stomach can be completely mobilized, preserving the gastroepiploic arcade, and the narrow gastric conduit can be fashioned as with routine cases. The Roux limb can be resected, re-anastomosed to the biliary-pancreatic limb, or used to create a jejunostomy, according to the surgeon’s preference (89,90). Finally, the gastric pouch and the distal esophagus should be mobilized into the mediastinum, and the gastric conduit sutured to the gastric pouch (88) for easy retrieval from the chest. The timing for esophagectomy has to be carefully planned—indeed, patients at early stage of esophageal cancer or with a reasonable response to treatment might undergo a weight-loss regiment first, thus offering esophagectomy when the lower BMI could decrease their post-operative morbidity and mortality risks. However, as previously mentioned, no studies have been published to define the best timing for those options in case weight-loss surgery and esophagectomy are the chosen therapeutic options.
Conclusions
Esophagectomy in obese patients with esophageal cancer is a safe and feasible procedure which leads to good oncological and clinical outcomes. A careful pre-operative multidisciplinary planning and a vigilant post-operative management are key elements to reduce morbidity and enhance recovery.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Fernando A. M. Herbella, Rafael Laurino Neto and Rafael C. Katayama) for the series “How Can We Improve Outcomes for Esophageal Cancer?” published in Annals of Esophagus. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoe.2020.02.02). The series “How Can We Improve Outcomes for Esophageal Cancer?” was commissioned by the editorial office without any funding or sponsorship. DM reports grants from Intuitive, other (consulting) from Urogen, other (consulting) from Johnson and Johnson, other (consulting) from Boston Scientific, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization. Key facts on obesity and overweight. 2018. Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight
- NCD Risk Factor Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016;387:1377-96. [Crossref] [PubMed]
- Nimptsch K, Pischon T. Body fatness, related biomarkers and cancer risk: an epidemiological perspective. Horm Mol Biol Clin Investig 2015;22:39-51. [Crossref] [PubMed]
- World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and oesphageal cancer. 2016 (Revised 2018). Available online: https://www.wcrf.org/sites/default/files/Oesophageal-cancer-report.pdf
- World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and pancreatic cancer. 2012 (Revised 2018). Available online: https://www.wcrf.org/sites/default/files/Pancreatic-cancer-report.pdf
- World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and liver cancer. 2015 (Revised 2018). Available online: https://www.wcrf.org/sites/default/files/Liver-cancer-report.pdf
- World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and colorectal cancer. 2017 (Revised 2018). Available online: https://www.wcrf.org/sites/default/files/Colorectal-cancer-report.pdf
- World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and breast cancer. 2017 (Revised 2018). Available online: https://www.wcrf.org/sites/default/files/Breast-cancer-report.pdf
- World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and endometrial cancer. 2013 (Revised 2018). Available online: https://www.wcrf.org/sites/default/files/Endometrial-cancer-report.pdf
- World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and kidney cancer. 2015 (Revised 2018). Available online: https://www.wcrf.org/sites/default/files/Kidney-cancer-report.pdf
- Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer – viewpoint of the iARC working group. N Engl J Med 2016;375:794-8. [Crossref] [PubMed]
- World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and cancers of the mouth, pharynx and larynx. 2018. Available online: https://www.wcrf.org/sites/default/files/Mouth-Pharynx-Larynx-cancer-report.pdf
- World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and stomach cancer. 2016 (Revised 2018). Available online: https://www.wcrf.org/sites/default/files/Stomach-cancer-report.pdf
- World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and gallbladder cancer. 2015 (Revised 2018). Available online: https://www.wcrf.org/sites/default/files/Gallbladder-cancer-report.pdf
- World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and ovarian cancer. 2014 (Revised 2018). Available online: https://www.wcrf.org/sites/default/files/Ovarian-cancer-report.pdf
- World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and prostate cancer. 2014 (Revised 2018). Available online: https://www.wcrf.org/sites/default/files/Prostate-cancer-report.pdf
- Ligibel JA, Alfano CM, Courneya KS, et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol 2014;32:3568-74. [Crossref] [PubMed]
- Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med 2003;348:1625-38. [Crossref] [PubMed]
- Vainio H, Kaaks R, Bianchini F. Weight control and physical activity in cancer prevention: international evaluation of the evidence. Eur J Cancer Prev 2002;11:S94-100. [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7. [Crossref] [PubMed]
- World Health Organization. GLOBOCAN 2018. Graph production: IARC. Available online: http://gco.iarc.fr/today
- Pohl H, Sirovich B, Welch HG. Esophageal adenocarcinoma incidence: are we reaching the peak? Cancer Epidemiol Biomarkers Prev 2010;19:1468-70. [Crossref] [PubMed]
- Imperial College London, Continuous Update Project Team Members. World Cancer Research Fund International Systematic Literature Review: the association between food, nutrition and physical activity and the risk of oesophageal cancer. 2015. Available online: https://www.wcrf.org/sites/default/files/oesophageal-cancer-slr.pdf
- Lagergren J. Influence of obesity on the risk of esophageal disorders. Nat Rev Gastroenterol Hepatol 2011;8:340-7. [Crossref] [PubMed]
- Spechler SJ, Fitzgerald RC, Prasad GA, et al. History, molecular mechanisms, and endoscopic treatment of Barrett's esophagus. Gastroenterology 2010;138:854-69. [Crossref] [PubMed]
- Spechler SJ, Souza RF. Barrett’s esophagus. N Engl J Med 2014;371:836-45. [Crossref] [PubMed]
- Morales CP, Souza RF, Spechler SJ. Hallmarks of cancer progression in Barrett's oesophagus. Lancet 2002;360:1587-9. [Crossref] [PubMed]
- Ryan AM, Duong M, Healy L, et al. Obesity, metabolic syndrome and esophageal adenocarcinoma: epidemiology, etiology and new targets. Cancer Epidemiol 2011;35:309-19. [Crossref] [PubMed]
- Lagergren J, Bergstrom R, Nyren O. Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Ann Intern Med 1999;130:883-90. [Crossref] [PubMed]
- Chow WH, Blot WJ, Vaughan TL, et al. Body mass index and risk of adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst 1998;90:150-5. [Crossref] [PubMed]
- Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol 2011;29:415-45. [Crossref] [PubMed]
- Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 2004;4:579-91. [Crossref] [PubMed]
- Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 2006;6:772-83. [Crossref] [PubMed]
- Rubenstein JH, Kao JY, Madanick RD, et al. Association of adiponectin multimers with Barrett’s oesophagus. Gut 2009;58:1583-9. [Crossref] [PubMed]
- Thompson OM, Beresford SA, Kirk EA, et al. Serum leptin and adiponectin levels and risk of Barrett’s esophagus and intestinal metaplasia of the gastroesophageal junction. Obesity 2010;18:2204-11. [Crossref] [PubMed]
- Doyle SL, Donohoe CL, Finn SP, et al. IGF-1 and its receptor in esophageal cancer: association with adenocarcinoma and visceral obesity. Am J Gastroenterol 2012;107:196-204. [Crossref] [PubMed]
- Donohoe CL, Doyle SL, McGarrigle S, et al. Role of the insulin-like growth factor 1 axis and visceral adiposity in oesophageal adenocarcinoma. Br J Surg 2012;99:387-96. [Crossref] [PubMed]
- Greer KB, Thompson CL, Brenner L, et al. Association of insulin and insulin-like growth factors with Barrett’s oesophagus. Gut 2012;61:665-72. [Crossref] [PubMed]
- Garcia JM, Splenser AE, Kramer J, et al. Circulating inflammatory cytokines and adipokines are associated with increased risk of Barrett's esophagus: a case-control study. Clin Gastroenterol Hepatol 2014;12:229-238.e3. [Crossref] [PubMed]
- Nimptsch K, Steffen A, Pischon T. Obesity and oesophageal cancer. Recent Results Cancer Res 2016;208:67-80. [Crossref] [PubMed]
- Lindkvist B, Johansen D, Stocks T, et al. Metabolic risk factors for esophageal squamous cell carcinoma and adenocarcinoma: a prospective study of 580,000 subjects within the Me-Can project. BMC Cancer 2014;14:103. [Crossref] [PubMed]
- Chen ZM, Xu Z, Collins R, et al. Early health effects of the emerging tobacco epidemic in China. A 16-year prospective study. JAMA 1997;278:1500-4. [Crossref] [PubMed]
- Freedman ND, Abnet CC, Caporaso NE, et al. Impact of changing US cigarette smoking patterns on incident cancer: risks of 20 smoking-related cancers among the women and men of the NIH-AARP cohort. Int J Epidemiol 2016;45:846-56. [Crossref] [PubMed]
- Uppot RN, Sahani DV, Hahn PF, et al. Impact of obesity on medical imaging and image-guided intervention. AJR Am J Roentgenol 2007;188:433-40. [Crossref] [PubMed]
- Uppot RN, Sheehan A, Seethamraju R. Obesity and MR imaging. In: MRI hot topics. Malvern: Siemens Medical Solutions USA, 2008.
- Ng A, Smith G. Gastroesophageal reflux and aspiration of gastric contents in anesthetic practice. Anesth Analg 2001;93:494-513. [PubMed]
- Smith G, Ng A. Gastric reflux and pulmonary aspiration in anaesthesia. Minerva Anestesiol 2003;69:402-6. [PubMed]
- Brady M, Kinn S, Stuart P. Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst Rev 2003;CD004423 [PubMed]
- Salem MR, Khorasani A, Saatee S, et al. Gastric tubes and airway management in patients at risk of aspiration: history, current concepts, and proposal of an algorithm. Anesth Analg 2014;118:569-79. [Crossref] [PubMed]
- Knoth S, Weber B, Croll M, et al. Anaesthesiologic Techniques for Patients at Risk of Aspiration. Anasthesiol Intensivmed Notfallmed Schmerzther 2019;54:589-602. [PubMed]
- Rubinstein I, Zamel N, DuBarry L, et al. Airflow limitation in morbidly obese, nonsmoking men. Ann Intern Med 1990;112:828-32. [Crossref] [PubMed]
- Pelosi P, Croci M, Ravagnan I, et al. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. Anesth Analg 1998;87:654-60. [PubMed]
- Salome CM, King GG, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol 2010;108:206-11. [Crossref] [PubMed]
- Bahammam AS, Al-Jawder SE. Managing acute respiratory decompensation in the morbidly obese. Respirology 2012;17:759-71. [Crossref] [PubMed]
- Bucklin BA, Fernandez-Bustamante A. Chapter: Obesity and anesthesia. In: Barash PG, Cullen BF, Stoelting RK, et al. Clinical anesthesia 7th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins, 2013:1274-93.
- Steier J, Lunt A, Hart N, et al. Observational study of the effect of obesity on lung volumes. Thorax 2014;69:752-9. [Crossref] [PubMed]
- Lugogo NL, Kraft M, Dixon AE. Does obesity produce a distinct asthma phenotype? J Appl Physiol 2010;108:729-34. [Crossref] [PubMed]
- Sutherland ER, Goleva E, King TS, et al. Cluster analysis of obesity and asthma phenotypes. PLoS One 2012;7:e36631 [Crossref] [PubMed]
- Al-Alwan A, Bates JH, Chapman DG, et al. The nonallergic asthma of obesity. A matter of distal lung compliance. Am J Respir Crit Care Med 2014;189:1494-502. [Crossref] [PubMed]
- Kaw R, Michota F, Jaffer A, et al. Unrecognized sleep apnea in the surgical patient: implications for the perioperative setting. Chest 2006;129:198-205. [Crossref] [PubMed]
- Garg R, Singh A, Prasad R, et al. A comparative study on the clinical and polysomnographic pattern of obstructive sleep apnea among obese and non-obese subjects. Annals of Thoracic Medicine 2012;7:26-30. [Crossref] [PubMed]
- Singh M, Liao P, Kobah S, et al. Proportion of surgical patients with undiagnosed obstructive sleep apnoea. Br J Anaesth 2013;110:629-36. [Crossref] [PubMed]
- Olson AL, Zwillich C. The obesity hypoventilation syndrome. Am J Med 2005;118:948-56. [Crossref] [PubMed]
- Chau EH, Lam D, Wong J, et al. Obesity hypoventilation syndrome: a review of epidemiology, pathophysiology, and perioperative considerations. Anesthesiology 2012;117:188-205. [Crossref] [PubMed]
- Pépin JL, Borel JC, Janssens JP. Obesity hypoventilation syndrome: an underdiagnosed and undertreated condition. Am J Respir Crit Care Med 2012;186:1205-7. [Crossref] [PubMed]
- Fernandez-Bustamante A, Hashimoto S, Serpa Neto A, et al. Perioperative lung protective ventilation in obese patients. BMC Anesthesiol 2015;15:56. [Crossref] [PubMed]
- Licker M, Diaper J, Villiger Y, et al. Impact of intraoperative lung-protective interventions in patients undergoing lung cancer surgery. Crit Care 2009;13:R41. [Crossref] [PubMed]
- Yang M, Ahn HJ, Kim K, et al. Does a protective ventilation strategy reduce the risk of pulmonary complications after lung cancer surgery?: a randomized controlled trial. Chest 2011;139:530-7. [Crossref] [PubMed]
- Maslow AD, Stafford TS, Davignon KR, et al. A randomized comparison of different ventilator strategies during thoracotomy for pulmonary resection. J Thorac Cardiovasc Surg 2013;146:38-44. [Crossref] [PubMed]
- Unzueta C, Tusman G, Suarez-Sipmann F, et al. Alveolar recruitment improves ventilation during thoracic surgery: a randomized controlled trial. Br J Anaesth 2012;108:517-24. [Crossref] [PubMed]
- Astle SM. Pain management in critically ill obese patients. Crit Care Nurs Clin North Am 2009;21:323-39. [Crossref] [PubMed]
- Smit C, De Hoogd S, Brüggemann RJM, et al. Obesity and drug pharmacology: a review of the influence of obesity on pharmacokinetic and pharmacodynamic parameters. Expert Opin Drug Metab Toxicol 2018;14:275-85. [Crossref] [PubMed]
- Ajani JA, D’Amico TA, Bentre DJ, et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2019;17:855-83. [Crossref] [PubMed]
- Bamgbade OA, Rutter TW, Nafiu OO, et al. Postoperative complications in obese and non-obese patients. World J Surg 2007;31:556-60. [Crossref] [PubMed]
- Melis M, Weber JM, McLoughlin JM, et al. An elevated body mass index does not reduce survival after esophagectomy for cancer. Ann Surg Oncol 2011;18:824-31. [Crossref] [PubMed]
- Scarpa M, Cagol M, Bettini S, et al. Overweight patients operated on for cancer of the esophagus survive longer than normal-weight patients. J Gastrointest Surg 2013;17:218-27. [Crossref] [PubMed]
- Zhang SS, Yang H, Luo JK, et al. The impact of body mass index on complication and survival in resected oesophageal cancer: a clinical-based cohort and meta-analysis. Br J Cancer 2013;109:2894-903. [Crossref] [PubMed]
- Mengardo V, Pucetti F, Mc Cormack O, et al. The impact of obesity on esophagectomy: a meta-analysis. Dis Esophagus 2018; [Crossref] [PubMed]
- Sgourakis G, Gockel I, Radtke A, et al. Minimally invasive versus open esophagectomy: meta-analysis of outcomes. Dig Dis Sci 2010;55:3031-40. [Crossref] [PubMed]
- Biere SSAY, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-lable, randomized controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Fenton JR, Bergeron EJ, Coello M, et al. Feeding jejunostomy tubes placed during esophagectomy: are they necessary? Ann Thorac Surg 2011;92:504-11; discussion 511-2. [Crossref] [PubMed]
- Kayani B, Okabayashi K, Ashrafian H, et al. Does obesity affect outcomes in patients undergoing esophagectomy for cancer? A meta-analysis. World J Surg 2012;36:1785-95. [Crossref] [PubMed]
- Ouattara M, D'Journo XB, Loundou A, et al. Body mass index kinetics and risk factors of malnutrition one year after radical oesophagectomy for cancer. Eur J Cardiothorac Surg 2012;41:1088-93. [Crossref] [PubMed]
- Martin L, Jia C, Rouvelas I, et al. Risk factors for malnutrition after oesophageal and cardia cancer surgery. Br J Surg 2008;95:1362-8. [Crossref] [PubMed]
- Martin L, Lagergren J, Lindblad M, et al. Malnutrition after oesophageal cancer surgery in Sweden. Br J Surg 2007;94:1496-500. [Crossref] [PubMed]
- Martin L, Lagergren P. Long-term weight change after oesophageal cancer surgery. Br J Surg 2009;96:1308-14. [Crossref] [PubMed]
- Rossidis G, Browning R, Hochwald SN, et al. Minimally invasive esophagectomy is safe in patients with previous gastric bypass. Surg Obes Relat Dis 2014;10:95-100. [Crossref] [PubMed]
- Marino KA, Weksler B. Esophagectomy after weight-reduction surgery. Thorac Surg Clin 2018;28:53-8. [Crossref] [PubMed]
- Nguyen NT, Tran CL, Gelfand DV, et al. Laparoscopic and thoracoscopic Ivor Lewis esophagectomy after Roux-en-Y gastric bypass. Ann Thorac Surg 2006;82:1910-3. [Crossref] [PubMed]
- Ellison HB, Parker DM, Horsley RD, et al. Laparoscopic transhiatal esophagectomy for esophageal adenocarcinoma identified at laparoscopic Roux-en-Y gastric bypass. Int J Surg Case Rep 2016;25:179-83. [Crossref] [PubMed]
Cite this article as: Amabile A, Carr R, Molena D. Esophagectomy for cancer in the obese patient. Ann Esophagus 2020;3:6.


