High-grade dysplasia of the esophagus: when is it time for surgery? —a systematic review
Introduction
Endoscopic management of high-grade dysplasia (HGD) of the esophagus, an alternative to esophagectomy, was first introduced in 1992 and has been recognized as standard of care since 2009 (1,2). Prior to that, esophagectomy was the standard of care due to association between malignancy and HGD among patients with Barrett’s esophagus (BE). Less than 1% per year of patients with BE will develop HGD but up to 40% of these patients will go on to develop invasive adenocarcinoma (3-5). Most patients with HGD are now manageable with a combination of endoscopic options; including endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), radiofrequency ablation (RFA), argon plasma coagulation (APC) and cryoablation (6-10). These treatments have become the preferred therapy for most patients with HGD or superficial esophageal cancer because they offer esophageal preservation, similar long-term survival, and significantly fewer complications compared with esophagectomy (11).
While endoscopic therapy is preferred over surgical therapy for HGD, guidelines for selecting which patient would benefit from surgical management are absent (12). Many surgeons believe that esophagectomy is more appropriate in individuals with poor esophageal body function, severe and/or uncontrollable reflux symptoms, dysphagia, or frequent aspiration (13). This systematic review aims to examine current literature to define the role of surgery in the treatment of HGD.
Data sources
English-language studies in persons older than 18 were identified by searching the MEDLINE database from January 2010 to December 2018. In 1992 EMR with fitted cap was first published by Dr. Inoue, however, endoscopic treatment of HGD was not an acceptable standard of care until 2009 (1,2). Therefore, only studies published after 2009 were considered.
Study selection
Three independent assessors reviewed all papers for inclusion. Key words used to identify articles included “esophageal high-grade dysplasia” and “surgery”. All identified articles were systematically assessed using the inclusion and exclusion criteria. The reference lists of articles matching inclusion criteria were then reviewed for further identification of potentially relevant studies. All retrieved citations were reviewed to identify all experimental, cohort, or case-control studies that compared treatment options and outcomes for patients with HGD.
Selection criteria
Studies in which patients had a diagnosis of HGD or Barrett’s dysplasia were included for review. Case reports and review articles were excluded. Only retrospective or prospective studies were included. After review of full text, additional studies were excluded if surgical intervention or referral to surgery was not evaluated or mentioned.
Data extraction
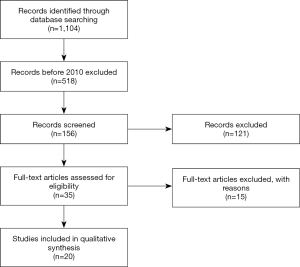
Article text, figures, and tables were reviewed. Three investigators (SG Worrell, KE Bingmer, A Ofshteyn) reviewed studies selected through exclusion and inclusions criteria. Discrepancies between the two reviewers were resolved by discussion until consensus achieved. There have been 1,104 papers published since 1992 and 518 since 2010. All 518 titles were reviewed and excluded using the selection criteria above. This including exclusion of case reports, basic science papers, and review articles. After this, there were 156 remaining papers. All abstracts of the 156 manuscripts were reviewed, 35 papers were selected to be individually reviewed. Final selection identified 20 papers (Figure 1) for inclusion.
Data synthesis
Among 35 studies that were fully reviewed, 20 papers mentioned both endoscopic therapy and surgery and were included in this review. Among the 20 studies, there were no randomized controlled trials, but in aggregate these studies included at least 781 patients with HGD alone and over 1,566 patients with either HGD or intramucosal adenocarcinoma (IMC).
There were only two studies which directly compared the outcomes of endoscopic and surgical treatment (Table 1) (14,15). Both of these studies were retrospective and included both HGD and IMC. Endoscopic eradication was achieved in 45–70% with a recurrence rate of 6–20% (14,15).
Table 1
| Article | Year | Pathology | Patients | Management | Complications | Progression to esophagectomy | Recurrence | Eradication using endoscopic technique | Mean follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Le Page ( |
2015 | HGD | 35 | Endoscopic (n=32) 91% | Endoscopic all 1 (2%) | 5 | 2 (6.3%) | 35 (70%) | 21 months |
| Surgical (n=3) 9% | |||||||||
| IMC | 48 | Endoscopic (n=18) 38% | Surgical all 24 (72.7%) | n/a | 1 (5.6%) | n/a | |||
| Surgical (n=30) 62% | |||||||||
| Zehetner ( |
2010 | HGD | 35 | Endoscopic (n=22) 63% | Endoscopic all 0 | 2 | 8 (20%) | 18 (45%) | 60 months |
| Surgical (n=13) 37% | n/a | ||||||||
| IMC | 66 | Endoscopic (n=18) 27% | Surgical all 24 (39%) | 1 | 0 | n/a | |||
| Surgical (n=48) 73% | n/a |
*, five patients underwent surgery following EMR, and are counted at EMR initially but surgical for complication and recurrence. EMR, endoscopic mucosal resection.
The remaining 18 studies were non-comparative, case-control studies. The indication for surgery was progression to cancer, failure to clear disease, or recurrence of HGD or IMC during surveillance after HGD had been cleared with an overall referral to surgery rate of 0 to 24%. Of these 18 articles, 14 discussed outcomes of HGD with varying intervention. The other four articles addressed cost effectiveness and surgical outcomes alone and are discussed in the appropriate sub-sections. Specific outcomes of the 14 studies are shown in Table 2, including surgery rate, clinical outcomes, and recurrence rate. These studies demonstrate some of the adverse outcomes associated with endoscopic treatment. There was a post-treatment stricture rate of 2% to 26%, which was higher among patients treated with the ESD technique. There was a recurrence rate of dysplasia of 3% to 21%, with the majority of recurrences occurring within the first two years following initial treatment. Overall 5-year survival rate was between 77% to 91% for all treatments. A proposed treatment algorithm with consideration of special topics, addressed below, is shown in Figure 2.
Table 2
| Therapy | N | Referred for surgery | BE length [IQR] in cm | Median number of treatments [IQR] | Complications | Progression to cancer | Stricture | Persistent dysplasia | Recurrence of HGD or IMC | Median follow-up (range or IQR) in months | 5-year survival |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cryotherapy | |||||||||||
| Ramay ( |
50 HGD and IMC | 1/50 (2%) | 3.5 [2–5] | 3 [2–5] | NR | 2/50 (4.0%) | NR | 4/50 (8.0%) | 8/49 (16%) at median 8 months | 64.6 | NR |
| Halsey ( |
68 HGD | NR | 3 [2–5] | NR | NR | 1/68 (1.5%) | NR | 0 | 6/36 (17%) at median 6.5 months | 24 (IQR, 11.6–29.3) | NR |
| RFA | |||||||||||
| Sengupta ( |
66 HGD out of 121 HGD, LGD, IMC, indefinite dysplasia | 4/121 (3.3%) | 4 [2–7] | 3 [1–12] | 3/121 (2.5%) | NR | 3/121 (2.5%) | 30/121 (24.8%) | 3/91 (3%) at median 12 months | NR | 99.2% |
| EMR +/− RFA | |||||||||||
| Yamashita ( |
14 HGD out of 28 HGD, LGD, IMC intermediate dysplasia | 4/28 (14.3%) | 2 [1–5] | 3.5 [2–5] | 2/28 (7.1%) | 1/28 (3.6%) | 2/11 (18.2%) | 3/14 (21.4%) | NR | 13.3 | NR |
| Small ( |
135 HGD, 111 IMC | NR | 2 [1–5] HGD, 3 [2–5] IMC | 2 [1–3] HGD, 3 [1–4] IMC | 1/72 (1.4%) | 1/135 (0.7%) | NR | 22/135 (16.3%) | 9/113 (8%) at median 18 months | 37 (IQR, 19–59) | 90.2% |
| Al Natour ( |
32 HGD, 28 IMC | 0 | 4 [2–7] | 1 EMR [1–2], 3 RFA [2–5] | 13/60 (21.7%) | 0 | 11/60 (18.3%) | 1/32 (3.1%) | 2/60 (3%) HGD | 33 (IQR, 16–50) | NR |
| Oliphant ( |
63 HGD, 9 IMC | 8/72 (11.1%) | NR | 4 [1–11] | 7/72 (9.7%) | 8/63 (12.7%) | 6/72 (8.3%) | NR | NR | 38 (range, 12 months–7 years) | NR |
| ESD | |||||||||||
| Zhang ( |
45 HGD out of 102 IMC or other SCC | 1/102 (1.0%) | NR | NR | 28/102 (27.5%) | 0 | 26/102 (25.5%) | 0 | 1/45 (2%), 3/102 (3%) | 33.6 (range, 3–81 months) | NR |
| Combination of ablation techniques +/− EMR | |||||||||||
| Yasuda ( |
103 HGD, 80 IMC | 3/183 (1.6%) | 133 BE ≥5 cm | NR | NR | 20/183 (10.9%) | 29/183 (15.8%) | NR | 20/183 (11%) at median 11.5 months | 39 (range, 5–179 months) | 77% |
| Guarner-Argente ( |
166 HGD, IMC and other dysplasia | 1/166 (0.6%) | 2 [1–5] | 2 [1–3] | 42/166 (25.3%) | 3/166 (1.8%) | 21/166 (12.7%) | 8/166 (4.8%) | 18/166 (11%) at median 16 months | 39 (range, 24–62) | 86% |
| Moss ( |
75 HGD or early adenocarcinoma | 7/75 (9.3%) | 3.6 [1–16] | 1 [1–5] | 7/75 (9.3%) | 6/70 (8.6%) | 6/75 (8.0%) | NR | 0 at mean 31 months | 31 (range, 3–89) | NR |
| Lada ( |
45 HGD, 12 IMC | NR | NR | 4 [1–11] | 2/57 (3.5%) | 4/57 (7.0%) | 1/57 (1.8%) | NR | 12/57 (21%) at median 29 months | 35.4 (range, 18.5–52.0) | NR |
| Hunt ( |
38 HGD and IMC | 9/38 (23.7%) | NR | NR | 4/38 (10.5%) | 8/38 (21.1%) | 2/38 (5.3%) | 0 | 3/38 (8%) at median 15.3 months | NR | NR |
| Qumseya ( |
52 HGD, 64 EAC | 19/116 (16.4%) | 3 [2–7] HGD, 2 [1–6] EAC | NR | 45/175 (25.7%) | 1/52 (1.9%) | 31/175 (17.7%) | 1/52 (1.9%) | 6/175 (3%) at median 11 months | 20 for HGD, 17 for early adenocarcinoma | 86% HGD, 78% EAC |
HGD, high grade dysplasia; BE, Barrett’s esophagus; IQR, interquartile range; IMC, intramucosal carcinoma; NR, not recorded; LGD, low-grade dysplasia; EMR, endoscopic mucosal resection; RFA, radiofrequency ablation; SCC, squamous cell carcinoma; EAC, esophageal adenocarcinoma.
Pathologist expertise
A potentially overlooked contraindication to endoscopic treatment of HGD and IMC is the absence of a reliable pathologist who can correctly interpret the endoscopic specimens as well as an endoscopist who can provide reliable specimens. In the paper by Small and colleagues, aiming to compare eradication of HGD and IMC with EMR +/− ablation, they found that 29% (54/189) of patients initially referred for therapy of BE with HGD had esophageal cancer. The majority had IMC, but 2% (4/189) had more invasive pathology, requiring esophagectomy (9). Furthermore, in another study of 67 patients referred with biopsies consistent with HGD, endoscopic resection (ER) specimens showed cancer in 13/67 (19%) with submucosal adenocarcinoma in 5/67 (7%) (21). The inter-observer variability is also acknowledged by the American College of Gastroenterology (ACG) guidelines who recommend any diagnosis of dysplasia of any grade should be reviewed by two pathologists, at least one with specialized expertise in gastrointestinal (GI) pathology (25).
Risk of endoscopic therapy failure
Patients with a large hiatal hernia and longer segment of BE are less likely to have complete eradication of dysplasia with RFA (8). In a cohort of 121 patients with dysplasia, 91 patients (75%) had complete eradication with a single treatment of RFA (8). There was recurrence of dysplasia in three patients (3%, 3/91). Among the 121 patients, 4 patients (3%) went on to esophagectomy due to persistent dysplasia. Incomplete eradication of dysplasia after RFA was associated with longer length of BE [7 cm (IQR, 4–10 cm) vs. 4 cm (IQR, 2–6 cm), P=0.004]. Hiatal hernia was also associated with incomplete eradication. A hiatal hernia was present in 83% of those without complete eradication and 55% of patients with complete eradication of dysplasia, P=0.005. Despite lack of detail regarding the eradication and hernia size in that study, other studies have suggested that hiatal hernia >4 cm, and histology of HGD or adenocarcinoma after initial ablation are significantly associated with recurrence of development of adenocarcinoma after ablation (20). Additionally, in a study of 90 patients who underwent EMR with ablation, only long segment BE was an independent predictor of recurrence of neoplasia or BE, odds ratio (OR) 2.73 with a 95% CI: 1.01–7.38 (26). Treatment failures in a study of 166 patients treated with EMR and ablation occurred in 8 patients (4.8%). All patients in whom complete eradication could not be achieved had multifocal HGD at initial treatment and 7 had long segment BE (≥5 cm). The HGD progressed to cancer in three cases (10). All identified risk factors for recurrence and failure of eradication are listed in Table 3. Both the exact size of hiatal hernia and length BE where endoscopy therapy fails is yet to be defined. However, these studies suggest a hiatal hernia >4 cm and BE length ≥5 cm were less likely to achieve complete eradication of dysplasia (8,10,20,26).
Table 3
| Factor | OR (95% CI) or success |
|---|---|
| Age ( |
1.10 (1.00–1.20), P=0.04 |
| 1.08 (1.01–1.15), P=0.032 | |
| Multifocal dysplasia ( |
3.22 (1.11–9.39), P=0.032 |
| 18% |
|
| Histology of HGD or adenocarcinoma after initial ablation ( |
4.14 (1.489–11.519), P=0.0065 |
| Hiatal hernia >4 cm ( |
3.649 (1.193–11.165), P=0.0233 |
| Long segment BE ( |
2.73 (1.01–7.38) |
| Nodular or ulcer ( |
39% |
HGD, high grade dysplasia; OR, odds ratio; CI, confidence interval; BE, Barrett’s esophagus.
Patient compliance with multiple treatments over a prolonged time period is necessary. The majority of patients need more than a single treatment for eradication of disease. In a study of 166 patients, 105 with HGD and 71 with intramucosal carcinoma, undergoing multiple modality endoscopic therapy, only 53/166 (32%) achieved complete eradication of dysplasia with a single treatment (10). In the same study, three patients had persistent HGD up to 50 months after initial endoscopic therapy (10).
There were no papers identified that evaluated outcomes of patients with poor motility, uuncontrollable reflux symptoms, dysphagia, or frequent aspiration. Therefore, no statement can be made regarding applicability of endoscopic intervention for these patients.
Esophagectomy after endotherapy
Confirming the findings associated with failed endotherapy, a multi-institutional study found that of those referred for surgery, 73% had long segment BE, 93% had a nodule or ulcer, and 91% had multifocal disease on initial endoscopy (27). Patients in this series underwent endotherapy for a mean of 13 months (range, 4–36 months) before referral for surgery. Patients were referred for surgery due to pathologic progression of disease (53%), failure to clear disease (33%), or recurrence of HGD or IMC during surveillance after it had been cleared (13%). Strictures developed in 20% of patients during endotherapy. On final surgical pathology four patients (27%) had submucosal cancer at resection and three of these patients (20% of the total group) had positive lymph nodes (LNs). These four patients were more likely to have IMC on initial endoscopy and underwent more endotherapy sessions. This study concluded that when the endoscopist is unable to eradicate the dysplastic epithelium after three sessions of RFA, the patient has IMC on index biopsy or nodular Barrett’s, and/or the patient develops a stricture during therapy, restaging and consideration of surgery should be discussed with the patient due to the lack of published guidelines (27).
Cost effectiveness
Endoscopic treatment, including continued surveillance, is cost effective over esophagectomy. Markov modeling demonstrated a cost savings of $21.8K for a 65-year-old patient treated with EMR and RFA combination therapy compared to esophagectomy (28). However, esophagectomy was found to be more cost effective for HGD variants that carry a 30% rate per year progression to cancer, which were identified as ulcerated, nodular, and diffuse HGD in the model. Additionally, surveillance, to identify patients early in their disease, would likely result in cost savings as lower stage esophageal cancer is less costly than advanced cancer requiring multi-modality treatment. Compared with current practice, potential incremental benefit is greatest for early detection in a BE surveillance program resulting in a gain of $4,971 by shifting 20% patients presenting with stage T3 cancer to HGD or T1 (29).
Surveillance
After endoscopic therapy recurrence can occur at prolonged time intervals. In a study of 90 patients with long-term follow-up recurrent dysplasia, after complete endoscopic eradication, occurred at a median of 44 months and up to 85 months post-treatment (26). The overall 5-year recurrence of HGD following complete eradication is 13.5% (9).
Survival
The 5-year survival for patients with HGD following successful endoscopic treatment appears equivalent to surgery (Table 2). Despite lack of comparative prospective trials, the overall survival for HGD treated with endoscopic therapy and surgery are similar. The 5-, 10- and 15-year survival following esophagectomy for HGD is 94%, 82%, and 75% (30). The 5- and 8-year survival following EMR +/− ablation is 90.2% and 79.9% (9).
Role/frequency of fundoplication
Only three papers mentioned the use of a fundoplication for reflux control. In all 20 papers combined, with over 781 HGD patients, there were six Nissens preformed pre-endoscopic therapy and 13 performed following endoscopic therapy. Notably, a majority of patients who underwent a fundoplication had their endoscopic treatments performed by a surgeon, rather than a gastroenterologist. No comparison or outcomes related to the use of fundoplication was reported (6,22,23).
Discussion
The diagnosis and management of HGD is an evolving and complex problem. Although Inoue introduced the concept of EMR in 1992, the use of endoscopic treatment for HGD and IMC was not popularized for another decade following the results of a study by Ell and colleagues where they showed successful endoscopic treatment with low morbidity and mortality (1,31). Endoscopic treatments have continued to evolve since being endorsed in 2009, and new technologies and techniques are continuing to be developed.
Although endoscopic treatments are often successful and allow for esophageal preservation, a subset of patients will require multiple treatments and/or have a high risk of disease recurrence. Certain patients, yet to be clearly defined, should be considered for esophagectomy as first-line treatment. In patients who have failed endoscopic therapy, esophagectomy should be considered as salvage treatment.
An esophagectomy is a complex operation with significant morbidity and mortality. Modern outcomes have decreased the incidence of mortality, which is now under 5% (32,33). There is still a major complication rate of 33%. The most common major complications include pneumonia, re-intubation, and anastomotic leak (33). Endoscopic therapy can be performed with minimal risks, although complications still exist. Serious adverse events following endoscopic therapy are reported in approximately 3% of patients with a stricture rate ranging from 3% to 26% following a combination of treatments. Additionally, stricture rate increases with the percentage of the resected circumference and the number of resected specimens (34).
Nonetheless, there are several issues with endoscopic treatment of HGD and IMC. A significant issue is whether there is appropriate expertise of the pathologist to differentiate HGD from IMC. One study found that community pathologists failed to identify invasive cancer in an HGD specimen 30% of the time (9). Additionally, when ER is performed there can be mis-interpretation of the specimen. In a multi-institutional study of 25 ER specimens there was an alarmingly high rate of discordance (48%) between study pathologists and original pathologic assessment (35). Pathologic diagnosis is what drives the decision algorithm. Therefore, understanding the institution-specific limitations of pathology programs will aid in making a successful endoscopic surveillance and treatment plan for patients with HGD.
The difficulties among expert pathologist to distinguish HGD from IMC and submucosal cancer has resulted in much of the literature combining high grade dysplasia and early esophageal cancer into one category (36). However, these are two intrinsically distinct disease processes. IMC is increasingly being treated with the same algorithms as HGD, receiving multiple endoscopic therapies prior to being considered for resection. However, IMC still carries a 3% risk of LN involvement (37,38). If IMC progresses to submucosal cancer during treatment, the risk of LN involvement goes up to 20%. Patients that have LN involvement have a 5-year survival rate of 45–67%—drastically reduced compared to 90–95% 5-year survival in patients with early esophageal cancer (39,40). There are well defined histologic characteristics that are associated with LN involvement: poor differentiation, lympho-vascular invasion, and multifocal HGD (41). When these features are seen on ER specimens, patients should be strongly considered for esophagectomy. While there is great evidence that low risk IMC can be cured by endoscopic therapy, future studies should separate these two disease processes to better define endoscopic outcomes for HGD and IMC individually.
Patients eligible for surgery who present with long segment BE (≥5 cm), multifocal dysplastic lesions, severe reflux symptoms, a large hiatal hernia, or dysphagia comprise a challenging group with regard to the appropriate treatment, either surgical or endoscopic. Other review articles have addressed the common perception that these patients would likely be better treated with esophagectomy. Specifically, long segment BE, large tumors, visible ulceration, and the presence of a hiatal hernia or prior fundoplication should warrant consideration of surgical over endoscopic treatment (42).
The majority of studies evaluating the effect of endoscopic treatments for HGD do not mention surgery. They continue to re-treat recurrences endoscopically and do not discuss timing of referral to surgery. Many of these studies exclude patients who progressed to cancer which, presumably is the time patients are referred for surgical resection. This gap in the literature begs the question: when is the time to stop endoscopic management and consider surgery for HGD? Hunt and colleagues suggest that when the endoscopist is unable to eradicate the dysplastic epithelium after three sessions of RFA, the patient has IMC on index biopsy or nodular Barrett’s, and/or the patient develops a stricture during therapy, restaging and consideration of surgery should be discussed with the patient due to the lack of published guidelines (27). These guidelines seem reasonable. However, there are no papers that have shown the number of treatments or that the presence of a stricture is associated with treatment failure. If patients understand the potential consequences of endoscopic therapy, then the number of treatments performed may be of no significance to their long-term outcomes. Additionally, strictures from endoscopic treatments may be superficial and treatable with dilation. In a study of patients undergoing photodynamic therapy (PDT) 94% of those that had developed post-PDT strictures were stricture-free at completion of the 2-year follow-up following multiple dilations (43). Due to the repeat nature of endoscopic therapy, patients who are not likely to be compliant with repeated endoscopy and surveillance should be considered for more definitive therapy with esophagectomy. This review identified multiple factors that were associated with treatment failure; nodules or ulcers, multifocal dysplasia, large hiatal hernias, long segment BE and persistent HGD after initial treatment (9,10,20,31).
Patients should additionally be counseled about the logistics of lifetime surveillance before undergoing endoscopic therapy for HGD. This is extremely necessary due to reports of recurrent dysplasia following endoscopic treatment out to 85 months (26). In a study that followed patients for a median of 6.8 years there was a 40% risk of recurrence of intestinal metaplasia and HGD following complete eradication of intestinal metaplasia (44). Of more concern, there is no decrease in the risk of recurrence over time (45). The ACG recommends surveillance after treatment of BE with HGD every 3 months for the first year, every 6 months for the second year and annually thereafter (25).
The population that still needs to be addressed are those patients diagnosed with HGD with concomitant severe reflux, motility disorders, or dysphagia. This group of patients are likely to have esophageal disease that may not respond as well to endoscopic treatment and is more appropriate for surgical resection, however, there are no studies that have specifically addressed this issue.
Another unanswered question is which endoscopic treatment option results in the best long-term outcomes with the lowest complication profile once HGD is identified. From this review, the combination therapies have the most success in regards to recurrence and survival with lower or equivalent stricture rates. Using just one therapy option and failing to completely eradicate dysplasia and intestinal metaplasia resulted in earlier recurrences. There continues to be advances in the options for endoscopic therapy and the ideal treatment may still be undefined. Regardless of the technique, it is clear that the key to success is complete eradication of intestinal metaplasia. Without complete eradication, there is a higher risk of recurrence of the BE, HGD, and IMC (46,47).
Additionally, beyond the scope of this review, but there is an increase in the role of fundoplication and endoscopic therapy. Patients in this review who underwent a fundoplication following endoscopic treatments are more likely to have their endoscopic treatment performed by a surgeon rather than a gastroenterologist. The ACG recommends that antireflux surgery should not be pursued in patients with BE as an antineoplastic measure. However, fundoplication should be considered in those with incomplete control of reflux symptoms on optimized medical therapy (25).
The main limitation of this study is it is a review of multiple studies with different selection criteria. Additionally, there are new treatment options becoming available as endoscopic management becomes more conventional. Some of these options may improve the outcomes for endoscopic therapy for HGD and eventually make surgery for HGD obsolete. Until this can be proven with lower recurrence rates there still remains a role for esophagectomy in this disease. Reviewing the differences in effectiveness of various endoscopic therapies outside the scope of this review. It was difficult to truly compare these patients as many of the endoscopic treatment studies excluded from their final analysis patients who needed surgery or had an incomplete ER. Another important limitation is that much of the literature combines HGD and IMC cancer in to one group, although we attempted to separate these as best as possible to examine only patients with HGD.
In conclusion, most patients are candidates for endoscopic intervention for HGD. Prior to beginning endoscopic therapy, all patients should be discussed in a multi-disciplinary setting. Combination therapy with varying ablation techniques and ER offers the best short-term outcomes, but carries a higher risk of recurrence, and these patients require long term surveillance. Patients with nodules or ulcers, multifocal dysplasia, large hiatal hernias, long segment BE, or persistent HGD after initial treatment should be counseled on their increased risk of recurrence and failure. These patients should be presented the risks and benefits of esophagectomy. Further, patients should understand the risk of stricture and disease progression as well as the need for lifelong surveillance and treatment prior to undergoing endoscopic therapy over esophagectomy for HGD.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoe.2020.01.01). CWT reports personal fees from Atricure, personal fees from Sig Medical, personal fees from Zimmer Biomet, personal fees and non-financial support from Medtronic, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Inoue H, Endo K, Takeshita K, et al. A new simplified technique of endoscopic esophageal mucosal resection using a cap-fitted panendoscope (EMRC). Surg Endosc 1992;6:264-5. [Crossref] [PubMed]
- Fernando HC, Murthy SC, Hofstetter W, et al. The society of thoracic surgeons practice guideline series: guidelines for the management of barrett’s esophagus with high-grade dysplasia. Ann Thorac Surg 2009;87:1993-2002. [Crossref] [PubMed]
- Jung KW, Talley NJ, Romero Y, et al. Epidemiology and natural history of intestinal metaplasia of the gastroesophageal junction and Barrett's esophagus: a population-based study. Am J Gastroenterol 2011;106:1447-55. [Crossref] [PubMed]
- Davila ML, Hofstetter WL. Endoscopic management of Barrett’s esophagus with high-grade dysplasia and early-stage esophageal adenocarcinoma. Thorac Surg Clin 2013;23:479-89. [Crossref] [PubMed]
- Shaheen NJ, Crosby MA, Bozymski EM, et al. Is there publication bias in the reporting of cancer risk in Barrett’s esophagus? Gastroenterology 2000;119:333-8. [Crossref] [PubMed]
- Yamashita DT, Li C, Bethune D, et al. Endoscopic mucosal resection for high-grade dysplasia and intramucosal carcinoma: a Canadian experience. Can J Surg 2017;60:129-33. [Crossref] [PubMed]
- Ramay FH, Cui Q, Greenwald BD. Outcomes after liquid nitrogen spray cryotherapy in Barrett's esophagus-associated high-grade dysplasia and intramucosal adenocarcinoma: 5-year follow-up. Gastrointest Endosc 2017;86:626-32. [Crossref] [PubMed]
- Sengupta N, Ketwaroo GA, Bak DM, et al. Salvage cryotherapy after failed radiofrequency ablation for Barrett's esophagus-related dysplasia is safe and effective. Gastrointest Endosc 2015;82:443-8. [Crossref] [PubMed]
- Small AJ, Sutherland SE, Hightower JS, et al. Comparative risk of recurrence of dysplasia and carcinoma after endoluminal eradication therapy of high-grade dysplasia versus intramucosal carcinoma in Barrett's esophagus. Gastrointest Endosc 2015;81:1158-66.e1-4.
- Guarner-Argente C, Buoncristiano T, Furth EE, et al. Long-term outcomes of patients with Barrett's esophagus and high-grade dysplasia or early cancer treated with endoluminal therapies with intention to complete eradication. Gastrointest Endosc 2013;77:190-9. [Crossref] [PubMed]
- Worrell S, DeMeester SR. Endoscopic Resection and Ablation for Early-Stage Esophageal Cancer. Thorac Surg Clin 2016;26:173-6. [Crossref] [PubMed]
- Bennett C, Vakil N, Bergman J, et al. Consensus statements for management of Barrett's dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology 2012;143:336-46. [Crossref] [PubMed]
- DeMeester SR. Evaluation and treatment of superficial esophageal cancer. J Gastrointest Surg 2010;14:S94-100. [Crossref] [PubMed]
- Le Page PA, Velu PP, Penman ID, et al. Surgical and endoscopic management of high grade dysplasia and early oesophageal adenocarcinoma. Surgeon 2016;14:315-21. [Crossref] [PubMed]
- Zehetner J, DeMeester SR, Hagen JA, et al. Endoscopic resection and ablation versus esophagectomy for high-grade dysplasia and intramucosal adenocarcinoma. J Thorac Cardiovasc Surg 2011;141:39-47. [Crossref] [PubMed]
- Halsey KD, Chang JW, Waldt A, Greenwald BD. Recurrent disease following endoscopic ablation of Barrett's high-grade dysplasia with spray cryotherapy. Endoscopy 2011;43:844-8. [Crossref] [PubMed]
- Al Natour RH, Catanzaro A, Zolotarevsky E, et al. Endoscopic therapy for Barrett's high grade dysplasia and intramucosal esophageal cancer is effective in community clinical practice by advanced endoscopists following multidisciplinary approach. Dis Esophagus 2018;31:1-6. [Crossref] [PubMed]
- Oliphant Z, Snow A, Knight H, et al. Endoscopic resection with or without mucosal ablation of high grade dysplasia and early oesophageal adenocarcinoma--long term follow up from a regional UK centre. Int J Surg 2014;12:1148-50. [Crossref] [PubMed]
- Zhang YQ, Chen T, Zhang C, et al. Endoscopic Submucosal Dissection for Superficial Proximal Esophageal Neoplasia is Highly Successful. Ann Surg 2017;266:995-9. [Crossref] [PubMed]
- Yasuda K, Choi SE, Nishioka NS, et al. Incidence and predictors of adenocarcinoma following endoscopic ablation of Barrett's esophagus. Dig Dis Sci 2014;59:1560-6. [Crossref] [PubMed]
- Moss A, Bourke MJ, Hourigan LF, et al. Endoscopic resection for Barrett's high-grade dysplasia and early esophageal adenocarcinoma: an essential staging procedure with long-term therapeutic benefit. Am J Gastroenterol 2010;105:1276-83. [Crossref] [PubMed]
- Lada MJ, Watson TJ, Shakoor A, et al. Eliminating a need for esophagectomy: endoscopic treatment of Barrett esophagus with early esophageal neoplasia. Semin Thorac Cardiovasc Surg 2014;26:274-84. [Crossref] [PubMed]
- Hunt BM, Louie BE, Schembre DB, et al. Outcomes in patients who have failed endoscopic therapy for dysplastic Barrett's metaplasia or early esophageal cancer. Ann Thorac Surg 2013;95:1734-40. [Crossref] [PubMed]
- Qumseya BJ, Panossian AM, Rizk C, et al. Survival in esophageal high-grade dysplasia/adenocarcinoma post endoscopic resection. Dig Liver Dis 2013;45:1028-33. [Crossref] [PubMed]
- Shaheen NJ, Falk GW, Iyer PG, et al. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol 2016;111:30-50. [Crossref] [PubMed]
- Anders M, Bähr C, El-Masry MA, et al. Long-term recurrence of neoplasia and Barrett's epithelium after complete endoscopic resection. Gut 2014;63:1535-43. [Crossref] [PubMed]
- Hunt BM, Louie BE, Dunst CM, et al. Esophagectomy for failed endoscopic therapy in patients with high-grade dysplasia or intramucosal carcinoma. Dis Esophagus 2014;27:362-7. [Crossref] [PubMed]
- Hu Y, Puri V, Shami VM, Stukenborg GJ, et al. Comparative Effectiveness of Esophagectomy Versus Endoscopic Treatment for Esophageal High-grade Dysplasia. Ann Surg 2016;263:719-26. [Crossref] [PubMed]
- Gordon LG, Hirst NG, Mayne GC, et al. Modeling the cost-effectiveness of strategies for treating esophageal adenocarcinoma and high-grade dysplasia. J Gastrointest Surg 2012;16:1451-61. [Crossref] [PubMed]
- Rice TW, Murthy SC, Mason DP, et al. Esophagectomy for clinical high-grade dysplasia. Eur J Cardiothorac Surg 2011;40:113-9. [Crossref] [PubMed]
- Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014;146:652-660.e1. [Crossref] [PubMed]
- Schieman C, Wigle DA, Deschamps C, et al. Patters of operative mortality following esophagectomy. Dis Esophagus 2012;25:645-51. [Crossref] [PubMed]
- Society of Thoracic Surgeons General Thoracic Surgery Database Task Force. The Society of Thoracic Surgeons Composite Score for Evaluating Esophagectomy for Esophageal Cancer. Ann Thorac Surg 2017;103:1661-7. [Crossref] [PubMed]
- Lewis JJ, Rubenstein JH, Singai AG, et al. Factors associated with esophageal stricture formation after endoscopic mucodal resection for neoplastic Barrett’s esophagus. Gastrointest Endosc 2011;74:753-60. [Crossref] [PubMed]
- Worrell SG, Boys JA, Chandrasoma P, et al. Inter-Observer Variability in the Interpretation of Endoscopic Mucosal Resection Specimens of Esophageal Adenocarcinoma: Interpretation of ER specimens. J Gastrointest Surg 2016;20:140-4; discussion 144-5. [Crossref] [PubMed]
- Goldblum JR. Controversies in the Diagnosis of Barrett Esophagus and Barrett-Related Dysplasia: One Pathologist's Perspective. Arch Pathol Lab Med 2010;134:1479-84. [PubMed]
- Leers JM, DeMeester SR, Oezcelik A, et al. The prevalence of lymph node metastases in patients with T1 esophageal adenocarcinoma a retrospective review of esophagectomy specimens. Ann Surg 2011;253:271-8. [Crossref] [PubMed]
- Nigro JJ, Hagen JA, DeMeester TR, et al. Prevalance and location of nodal metastases in distal esophageal adenocarcinoma confined to the wall: implications for therapy. J Thorac Cardiovasc Surg 1999;117:16-23. [Crossref] [PubMed]
- Molena D, Schlottmann F, Boys JA, et al. Esophagectomy Following Endoscopic Resection of Submucosal Esophageal Cancer: a Highly Curative Procedure Even with Nodal Metastases. J Gastrointest Surg 2017;21:62-7. [Crossref] [PubMed]
- Reed MF, Tolis G Jr, Edil BH, et al. Surgical treatment of esophageal high grade dysplasia. Ann Thorac Surg 2005;79:1110-5. [Crossref] [PubMed]
- Boys JA, Worrell SG, Chandrasoma P, et al. Can the risk of lymph node metastases be gauged in endoscopically resected submucosal esophageal adenocarcinomas? A multi-center study. J Gastrointest Surg 2016;20:6-12. [Crossref] [PubMed]
- McLaren PJ, Dolan JP. Surgical Treatment of High-Grade Dysplasia and Early Esophageal Cancer. World J Surg 2017;41:1712-8. [Crossref] [PubMed]
- Overholt BF, Wang KK, Burdick JS, et al. Five-year efficacy and safety of photodynamic therapy with Photofrin in Barrett's high-grade dysplasia. Gastrointest Endosc 2007;66:460-8. [Crossref] [PubMed]
- Schwameis K, Zehetner J, Green KM, et al. Workload, recurrence, quality of life and long term efficacy of endoscopic therapy for high-grade dysplasia and intramucosal esophageal adenocarcinoma. Ann Surg 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Sami SS, Ravindran A, Kahn A, et al. Timeline and location of recurrence following successful ablation in Barrett’s oesophagus: an international multicenter study. Gut 2019;68:1379-85. [Crossref] [PubMed]
- Sawas T, Alsawas M, Bazerbachi F, et al. Persistent intestinal metaplasia after endoscopic eradication therapy of neoplastic Barrett's esophagus increases the risk of dysplasia recurrence: meta-analysis. Gastrointest Endosc 2019;89:913-925.e6. [Crossref] [PubMed]
- Manner H, Rabenstein T, Pech O, et al. Ablation of residual Barrett’s epthelium after endoscopic resection: a randomized long-term follow-up study of argon plasma coagulation vs. surveillance (APE study). Endoscopy 2014;46:6-12. [PubMed]
Cite this article as: Worrell SG, Bingmer KE, Ofshteyn A, Towe CW, Perry Y, Linden PA. High-grade dysplasia of the esophagus: when is it time for surgery? —a systematic review. Ann Esophagus 2020;3:5.